Postnatal growth retardation (PGR) has a high incidence during early postnatal development of piglets and humans, inducing long-term impacts on development and a higher risk for adulthood metabolic syndromes. However, the mechanism of PGR remains unclear.
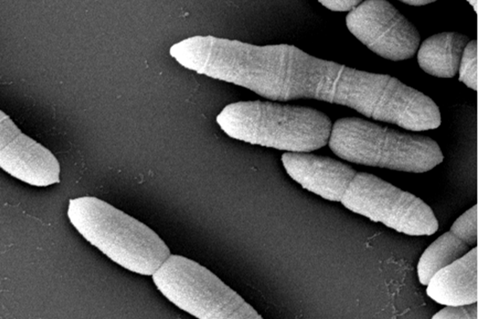
Gut microbiota is crucial in early-life development in humans and animals, but microbiome-induced host growth retardation remains incomplete. In a study published in Science Bulletin, researchers from Hunan Agricultural University found that hindgut-enriched Methanobrevibacter smithii compromises the weight gain in the pig PGR model. This finding provides new insight into the contributions of abnormal microbiota to growth retardation.
As one of the most important livestock species in the world, pigs are an important source of meat and serve as a good bio-model for research on human diseases. During the early life of pigs, postnatal growth retardation (PGR) with low growth rate is associated with metabolic disorders, leading to high mortality in pig production. Moreover, PGR also increases feed disappearance by 10%–30% and production cost.
In humans, PGR affects over 200 million children globally and accounts for 45% of mortality in children under 5 years. It has long-term impacts on child development and leads to a higher risk for various adulthood metabolic syndromes.
Multiple factors
Numerous factors, ranging from abnormalities in nutrient absorption to gut dysfunction, systemic inflammation, and decreased host energy utilization, have been invoked as contributors to the occurrence and development of PGR in children and animals, all of which can be impacted by the gut microbiome.
The researchers first investigated the differential growth performance of normal growth (Con) piglets and PGR piglets. Compared to Con piglets, the body weight of PGR piglets was significantly lower from the first week of life to day 60.
They performed hindgut microbial sequencing and found Methanogenic archaea belonging to Methanobrevibacter, and microbial pathways involved in methanogenesis were the strongest enrichment in the hindgut of PGR piglets.
Key metabolites
To identify key metabolites by which the gut microbiota influences host growth rate, they targeted a panel of host metabolites. Among differential metabolites, β-hydroxybutyrate (BHB), which is one of the main ketone bodies, was significantly depleted in PGR piglets and negatively associated with M. smithii abundance.
Moreover, they observed a hepatic-enriched inflammation-associated pathway in the PGR phenotype using RNA-seq as compared with Con. When the fecal microbiome from PGR piglets was transplanted to mice, they also found the same phenotypes in mice as shown in PGR piglets.
They further administered mice with M. smithii and observed the lower hepatic BHB level and liver inflammation as evidenced by the higher Kupffer M1 ratio. When the key ketogenesis gene in hepatocytes, Kupffer cells were prone to developing M1 features. Furthermore, they treated PGR piglets and M. smithii-induced PGR mice with BHB and found the significantly improving effects of BHB on growth performance. This study established a basis for the development of microbiome-targeted prevention and treatment strategies for growth retardation.





No comments yet