Researchers from Niigata University and Kyoto Prefectural University have revealed that small vesicles, around 100 nm in size, released by intestinal bacteria induce immune activation and progression of liver cirrhosis, as well as reduction of serum albumin level, subsequently leading to oedema and ascites.
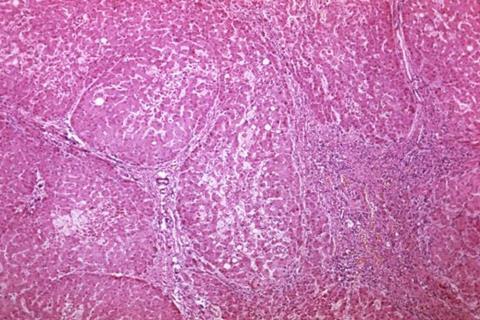
The global prevalence of cirrhosis is high and it can be fatal upon progressing to end-stages. The progression of cirrhosis results in various symptoms including jaundice, ascites, rupture of varices, and hepatocellular carcinoma. Often, even if root causes, such as hepatitis virus, alcohol, and lifestyle factors, are resolved, cirrhotic livers have limited regeneration capacity and the fibrosis progresses without improvement - this state is called the “point of no return.”
Intestinal bacteria are considered one of the important causes leading to this state. According to previous reports and clinical observations, it has been found that during cirrhosis, the intestine is susceptible to intestinal bacterial invasion, due to multiple factors such as oedematous mucosa and decrease of intestinal bacterial diversity, intestinal motility, mucus production, epithelial barrier function, and immune capacity.
Researchers have recently studied the possibility that the invasion of extremely small vesicles (around 100 nm), originating from certain intestinal bacteria, have an adverse effect on the liver without the invasion of the bacteria themselves.
Release of vesicles
In their experiments, the effects on the liver cells and cirrhosis model mice were analyzed when small vesicles were released by Escherichia coli (E. coli), which have been detected when the pathological state of cirrhosis worsens.
The results showed that vesicles derived from E. coli induced inflammation involving macrophage and neutrophils, and upregulated the expression of Clec4E gene (macrophage-inducible C-type lectin: Mincle) in the cells. Additionally, hepatocytes, which are the most important liver cells and responsible for the majority of the liver’s function, were found to undergo important changes when exposed to the vesicles, including decreased production of albumin, which is produced by hepatocytes and released into the blood.
Furthermore, administration of the vesicles derived from E. coli to cirrhosis model mice, induced inflammation of the liver, worsened fibrosis, and reduced serum levels of albumin. Moreover, these st)ates of activated inflammation and worsening fibrosis were alleviated by administering albumin to the mice. It was also found that the Clec4e gene was upregulated in the macrophages that had migrated from outside the liver.
Critical effects
The researchers also reported that vesicles derived from intestinal bacteria and multiple antibodies against various bacterial antigens were detected in the ascites and serums, respectively, of patients with decompensated cirrhosis. Under experimental scrutiny, the presence of these vesicles suggested the possibility that they had led to increased liver inflammation, worsening fibrosis, and decreased albumin production, causing critical effects in these patients.
The results of this study revealed the mechanism by which patients with advanced cirrhosis are exposed to chronic inflammation caused by bacterial vesicles and other agents, resulting in both liver and systemic damage. This phenomenon may be related to the mild inflammation and fever seen routinely in patients with cirrhosis.
Dr. Atsunori Tsuchiya of the Division of Gastroenterology and Hepatology, Graduate School of Medical and Dental Sciences, Future Medical Research Center for Exosome and Designer Cell (F-EDC), Niigata University, who led this project commented: “We will try to investigate the possibility of developing new therapies, for example, to strengthen the intestinal barriers of patients with cirrhosis.”





No comments yet