As we recover globally from the COVID-19 pandemic, the world is already amidst a bigger pandemic, albeit a silent one – antimicrobial resistance (AMR).
AMR – The Silent Global Pandemic
Microbes such as bacteria evolve rapidly to protect themselves from antimicrobial drugs that humans have created to target them. Infections caused by such microbes can be notoriously difficult to treat and can leave the patient with a slew of side effects from the long term courses of the drugs meant to treat them.
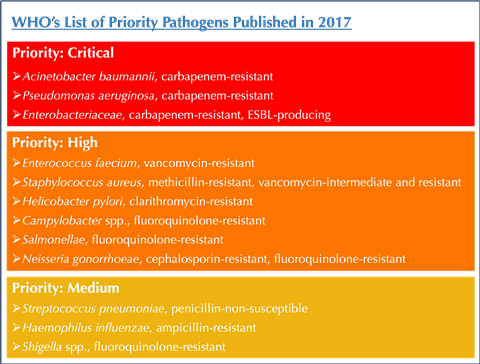
Antibiotic resistance in bacterial pathogens is rising at an alarming rate around the world. Bacterial infections, against which the fight seemed all but won over half a century ago when antibiotics became widely available, are once again the cause of significant morbidity and mortality. In 2017, the World Health Organisation (WHO) identified a list of 12 ‘priority pathogens’ – bacterial superbugs that have become resistant to the strongest of antibiotics that could be used against them and that pose the greatest threat to human health.
As per the 2016 O’Neill Review on Antimicrobial Resistance, it is estimated that by the year 2050, 10 million lives will be lost to AMR, a death rate higher than cancer. A recent paper published in The Lancet found that there were an estimated 1.27 million deaths attributable to antibiotic resistance in the year 2019 alone. This impact of AMR has only worsened after the COVID-19 pandemic, due to widespread use of antibiotics during the multiple waves of the infection. In its 2023 briefing on the global burden of AMR, the WHO declared AMR as one of the top 10 global health threats facing humanity with staggering potential impacts on the number of deaths from previously treatable infections, increase in healthcare costs, reduction in poultry numbers, among other factors.
Antibiotic Resistance – The India Perspective
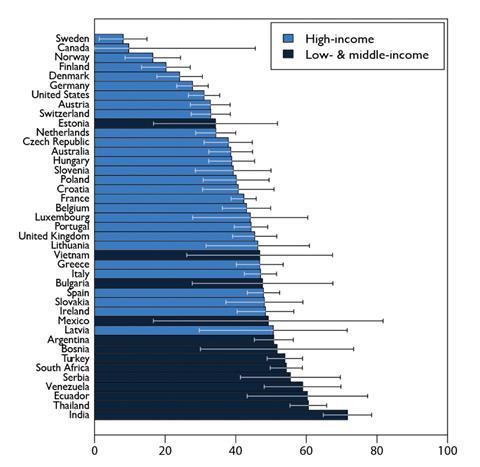
We zoom in on India, where antibiotic resistance is rampant. Studies conducted by the Indian Council of Medical Research (ICMR) indicate a sustained increase in antibiotic resistance in the country, at a rate of 5-10% each year. Susceptibility of pathogenic strains of bacteria to some of the strongest available antibiotics like carbapenems is falling at an alarming pace. As more and more people become infected with these extremely drug resistant (XDR) pathogens, their hope for recovery, and sometimes even survival, diminishes. The situation is made worse by the rampant and unchecked overuse of antibiotics like Colistin in agriculture, animal husbandry, and poultry farming industries, and the easy over-the-counter availability of antibiotics through pharmacy retailers around the country. As per the State of the World’s Antibiotics 2021 report, India has the highest levels of antibiotic resistance in the world.
Phage Therapy – A Credible Alternative to Antibiotics
With the global pipeline for new antibiotics being dry, the need for credible, evidence-backed and scientifically sound alternatives to combat bacterial infections is the need of the hour. Phage therapy is one such treatment option gaining ground in the last few years. Bacteriophages, or simply phages, are naturally occurring bacterial viruses that can attack and destroy bacterial colonies, including bacterial biofilms. Phages are present everywhere; they are the most abundant biological entity in the world, outnumbering bacteria by a factor of approximately 10 to 1. Phages can be of two kinds – virulent or ‘lytic’ phages, and temperate or ‘lysogenic’ phages. Lytic phages lyse the bacterial cell, infect the bacterial DNA to rapidly make hundreds of copies of themselves, destroy the bacterial cell, and release the progeny phages into the rest of the bacterial colony. Phages are extremely specific to their bacteria and will only infect the bacterial strain against which they are active. Phage therapy is the use of purified lytic phages for treatment of bacterial infections.
Our Journey – From Phage Therapy Patient to Advocate and Entrepreneur
In the spring of 2016, I found myself confronted with an antibiotic resistant infection. The urologist diagnosed my condition to be chronic bacterial prostatitis, an inflammation of the prostate gland typically caused by a bacterial infection. I was prescribed five antibiotics – Azithromycin, Doxycycline, Ofloxacin, Ciprofloxacin and IV Amikacin – to treat this condition over a period of 10 weeks. However, despite these varied and heavy doses of antibiotics, my infection persisted, and my symptoms of low-grade fever, chills, frequent urination, urinary urgency, right flank pain and lower back pain did not show any signs of improvement. At the end of my final course of antibiotics, a combination of oral Ciprofloxacin and IV Amikacin, I continued to have debilitating symptoms from the prostatitis condition, along with side effects from the heavy doses of antibiotics. The doctor declared the infection to be multidrug resistant and asked me to consider my condition to be one that would persist for the rest of my life. I was given a regimen of anti-inflammatory drugs, alpha-blockers, and analgesics to cope with my symptoms, and was advised to be on this regimen for the foreseeable future. At the age of 33, it was unacceptable for me to have to live with this condition that was causing me such severe symptoms and reducing the quality and scope of my life so significantly. After struggling with the hopelessness of suffering from an infection that was not responding to all known forms of medicine, my wife Apurva and I decided to investigate alternative forms of treatments for bacterial infections that do not respond to antibiotic therapy.
A long and hard search led us to discover ‘phage therapy’, a century-old treatment for bacterial infections, which was discovered and developed in the 1920s. Our research pointed us in the direction of the Eliava Institute for Bacteriophage, Virology and Microbiology located in Tbilisi, Georgia, as the oldest and most experienced practitioners of phage therapy in the world. In November 2016, we travelled to the Eliava Institute for my first course of phage treatment, and I became the first patient from India to undergo phage therapy at the Eliava Institute. Over the next 15 months, I underwent a total of three courses of phage therapy at the Eliava Institute’s phage clinic, at the end of which my multi-bacterial prostate infection had been cleared and the leukocyte counts in my urogenital samples were in the normal range. My symptoms progressively resolved through the three courses of phage therapy, and I was completely symptom-free by the end of my treatment.
The hopelessness that we had felt before learning of phage therapy drove us to establish Vitalis Phage Therapy, the first of its kind initiative in India with the aim to ensure that no one else who is suffering from a chronic, persistent, or recurrent infection and has run out of treatment options with antibiotics, ever needs to struggle in finding and accessing phage therapy.
The first step Vitalis took in 2018 was to work with the Eliava Phage Therapy Center to establish a remote treatment framework for India to enable local phage treatment for patients in India. Phage therapy is currently available to Indian patients on compassionate grounds on an individual basis, known as compassionate Phage Therapy (cPT), when all known interventions have failed for the patient. This is as per the World Medical Association’s Declaration of Helsinki, a set of ethical principles governing medical research on human subjects. India’s medical regulatory body, the Central Drugs Standard Control Organisation (CDSCO), has laid out the guidelines for import of small quantities of drugs otherwise not regulated in India, solely for personal use, accompanied by the prescription of a medical doctor registered to practice medicine in India. Enabling this crucial step eliminated the need for patients to travel internationally to undertake phage treatment.
In 2020, Vitalis established diagnostic phage sensitivity testing in India with phages from the Eliava Institute. Patients can now have their samples tested for phage sensitivity at a designated lab in India, something that could earlier only happen at the diagnostic lab of the Eliava Institute and involved increased costs and turnaround time (TAT) associated with shipping samples internationally. This step was taken keeping in mind patients who need phage therapy more urgently. With over 400 samples tested locally in the last three years since phage sensitivity testing began in India, this is a step that has benefitted each patient from India who has taken phage therapy from 2020 onwards.
Most recently, over the last two years, Vitalis is working with doctors and medical institutions across India to facilitate phage therapy for their patients suffering from antibiotic-resistant and hospital-acquired infections. Since Vitalis was established in early 2018, more than 200 patients suffering from antibiotic-resistant infections have been able to take phage therapy with a success rate of more than 70%. The conditions for which we receive the maximum number of queries for phage therapy from patients and doctors are chronic urinary tract infections (UTIs), prostatitis, surgical wound infections, and recurrent respiratory infections.

Regulatory Landscape for Phage Therapy
While the present regulations in India allow for individuals to undertake phage therapy, the logistical complexity involved with each case of cPT makes it a treatment option often outside of the reach of those who may need it most urgently. In recent years, there has been establishment of regulatory frameworks for phage therapy around the world to create wider access to it in the form of cPT, and to try to take it beyond compassionate treatment at least for certain conditions that are most impacted by the MDR / XDR infections. The Magistral Phage Act 2018 in Belgium provides special accreditation to laboratories to certify phage medical preparations as Magistral Phage, which can then be used for patient treatments as cPT. The Queen Astrid Military Hospital in Brussels has used this framework for treating over a 100 hundred patients with phage therapy. The Phage Australia consortium of 20 hospitals, research institutions, and laboratories, has succeeded in establishing the STAMP – Standardized Treatment and Monitoring Protocol for adults and paediatric patients. This provides standardised clinical protocols for individual cPT case, and with STAMP registered as a clinical trial, data collected for each cPT patient contributes towards the trial.
As regulatory bodies in various countries have begun to recognise and create regulations for phage therapy to enable wider access to it, the ICMR in India has also recognised the significant and critical role that phage therapy can play in combatting the AMR pandemic. A start has been made recently, with the ICMR inviting phage scientists and phage industry specialists to work together to establish a centre for phage research and application of phage therapy in India.
The road will be long for the potential of phage therapy to be well understood and for its use to be regulated by authorities in India and around the world. However, early steps have been taken, and we are hopeful that the course to be charted will happen at a pace that ensures that phage therapy can soon be an accessible form of treatment for patients suffering from antibiotic resistant, chronic, or recurrent infections around the world.








No comments yet