I’ll begin this article with a question that we’ll return to. Do microorganisms exhibit intelligence? Whilst it might not be able to gauge intelligence in single-celled organisms, a certain intelligent behaviour can be perceived. They possess the simplest chemical signalling pathways, yet are sophisticated enough to evade potential harm.
Bacteria in particular demonstrate remarkable capabilities, such as rapidly evolving and adapting to changing environments, including the development of antibiotic resistance and protection in the face of resource scarcity. A form of rudimentary learning and memory protects them, either from previously being exposed to harmful stimuli, or in utilising their past experiences to optimise resource acquisition. In the last few years, globally, there has been a growing concern about bacteria’s intelligent behaviour towards antibiotics – their resistance to a wide spectrum of antibiotics is a major health challenge.
To demonstrate this intelligence in bacteria, we can analyse the well-known Helicobacter pylori. This gram-negative, microaerophilic bacterium colonises the gastric mucus overlying the epithelium of the stomach. This genus of bacteria has been notorious for resisting numerous antibiotics, exasperating the growing global exigency in the fight against AMR.
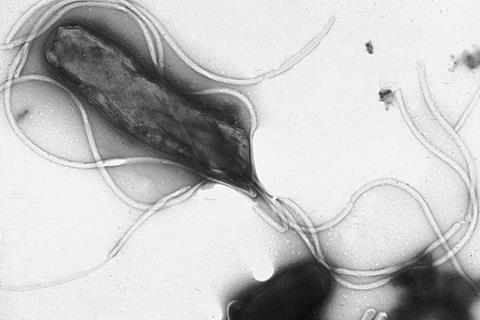
Helicobacter pylori
H. pylori finds itself capturing the third spot in the ‘Priority High category of the World Health Organization’s (WHO) list of antibiotic-resistant priority pathogens (bacteria that pose a significant threat to human health due to their resistance to multiple antibiotics). Sadly, Helicobacter pylori infects approximately 50% of the world’s population and is considered to be the major etiological agent of severe gastric diseases, such as peptic ulcers and gastric carcinoma. Infection primarily occurs from person-to-person contact, especially in crowded housing conditions, and may occur by drinking water contaminated with H. pylori.
The lack of a readily available vaccine against H. pylori is a significant concern, considering the widespread nature of the infection and its potential complications. People living in poverty and those in certain racial, ethnic, and immigrant groups are disproportionately affected by H. pylori infection. Despite the impact of treatment of infected individuals, and the reduced transmission of infection in communities in which socioeconomic living standards have improved, H. pylori continues to be the most common human bacterial pathogen, infecting perhaps half of the world’s population. As a result, it is still a major cause of morbidity and mortality worldwide.
What’s worse is that when treatment towards H. pylori fails, it leads to delays in eradicating the infection, providing more time for inflammation, potential progression to precancerous stages and ultimately advanced-stage cancer, which is heavily linked with poor treatment outcomes and fatality. It’s important to note that while AMR alone doesn’t directly cause cancer, it creates a challenging scenario where treating the infection becomes difficult, ultimately allowing the inflammation to persist and potentially contribute to cancer risk.

Previous estimates state that there were approximately 4.4 billion individuals with H. pylori infection worldwide in 2015. Incidence was highest in Nigeria.
Factfile: H. pylori
1. Global prevalence:
- H. pylori infects roughly half of the world’s population, with higher rates in developing countries.
- This translates to billions of people harbouring the bacteria, even if they are asymptomatic.
2. Potential complications:
While most infected individuals remain symptom-free, H. pylori can lead to severe health issues, including:
- Peptic ulcers: Painful sores in the stomach or duodenum, affecting millions globally.
- Gastritis: Inflammation of the stomach lining, causing discomfort and potential bleeding.
- Stomach cancer: Chronic H. pylori infection is a major risk factor for this highly lethal cancer
3. Increased challenges:
- Antibiotic resistance: H. pylori is becoming increasingly resistant to common antibiotics, making effective treatment more difficult.
- Limited treatment options: Current treatment regimens involve multiple medications, increasing compliance challenges and potential side effects.
4. Impact on healthcare systems:
H. pylori-related complications place a substantial burden on healthcare systems worldwide, resulting in:
- Increased healthcare costs;
- Reduced quality of life for affected individuals;
- Potential economic losses due to the illness and disability.
5. Urgency for solutions:
- The absence of a vaccine leaves significant gaps in prevention and control strategies.
- Continued research and development are crucial to address this concern.
6. Emerging areas of research:
While a widely available vaccine remains elusive, there are promising avenues for future interventions:
- Improved diagnostics: Faster and more accurate detection methods can facilitate early intervention.
- Alternative therapies: Exploring non-antibiotic approaches to eradicate H. pylori.
- Advanced research: Continued efforts towards developing effective vaccines remain ongoing.
7. A call to action:
- Raising awareness about H. pylori, its complications, and the need for effective solutions is crucial.
- Supporting research efforts and advocating for increased funding could expedite progress towards better prevention and control strategies.
A double whammy: cancer and antibiotic-resistant H. pylori
Chronic H. pylori infection is a major risk factor for stomach/gastric cancer. In the 15th Report on Carcinogens, a cumulative report mandated by US Congress and prepared by the National Toxicology Program (NTP) for the Secretary of the U.S. Department of Health and Human Services, chronic infection with the bacterium H. pylori was included in the list of human carcinogens. Finding a place in the carcinogen’s list makes it obvious that the potency of the bacterium is high, and strongly indicates the increased dangers it offers in elevating the risk of cancer.

Cancer as a result of H. pylori infection is usually caused by treatment failure, which allows resistant bacteria to persist and spread to others, further increasing the AMR problem. While H. pylori itself doesn’t directly cause cancer, the chronic inflammation it triggers damages the stomach lining. When the ongoing inflammation persists, the risk of further damage and eventually, cancer development increases.
Among several research studies on the association between cancer and H. pylori, one significant area of interest to many is the relationship between Immune Checkpoint Inhibitors (ICIs) and H. pylori. While ICIs have revolutionised cancer treatment by stimulating the immune system to attack tumours, their interaction with H. pylori infection is considered to be multifaceted.
Studies suggest that H. pylori infection might negatively impact the effectiveness of ICIs in certain cancer types. Other research indicates worse overall survival and disease progression in H. pylori-positive patients receiving ICI therapy compared to negative ones. In several other studies, however, researchers report mixed or even positive effects of H. pylori in condition-specific contexts. For example, in certain cases, the bacteria appear to stimulate anti-tumour immunity, potentially enhancing ICI benefit. To broaden the understanding of ICI in the context of H. pylori, further studies are crucial, throwing open the scope for plenty of research.
There are many ongoing research studies to explore the specific genetic variations associated with increased cancer risk due to H. pylori infection, which may pave the way for personalised screening and treatment strategies. Researchers are even exploring ways to neutralise or disrupt specific molecules produced by H. pylori that contribute to inflammation and cancer development.
Studying the complex interactions between H. pylori and other gut bacteria could lead to new approaches involving probiotics or dietary modifications to reduce cancer risk. With the evolution of advanced digital technology, algorithms are being used to analyse vast datasets of patient information, potentially leading to earlier detection and more effective treatment of H. pylori-related cancers.
New and emerging treatments for H. pylori infection
In countries like Japan, China, Korea, and Taiwan, researchers are channeling their R&D efforts to circumvent the problems caused by H. pylori. The bacterium has an increasing tendency to resist standard antibiotics, which has now led to a decreasing efficacy of eradication therapies. Reports indicate that available antibiotic therapies for H. pylori fail in up to 40% of patients. Hence, the research and development of novel and improved regimens for treatment are urgently required. Attributes driving this resistance mainly include mutations encoded chromosomally, but also the body’s physiological changes such as impaired regulation of drug uptake and/or efflux, and biofilm and coccoid formation.
“Treatment of H. pylori requires multidrug regimens because the organism resides in a layer of mucus that acts as a barrier to antibiotic penetration. Resistance is also an issue with some of the commonly used antibiotics: metronidazole, amoxicillin, erythromycin, and clarithromycin.”
When H. pylori develops resistance to commonly-used antibiotics the first-line treatment regimen becomes ineffective. This requires switching to alternative therapy, which is considered to be less effective. Usually, second-line therapies have lower success rates compromising eradication efforts.
In addition, these alternative drugs are often more expensive and require longer durations, placing a financial burden on patients. Alternatives to antibiotics also come with a higher risk of side effects, potentially discouraging patients from completing the treatment until cured. Researchers, around the world, have made substantial progress over the past few years in the identification of the bacterium’s molecular mechanisms, which have incited some efficient strategies to counteract resistance and to avoid the use of ineffective antibiotics. These involve molecular testing methods and the discovery of novel and potent antimicrobial compounds.

Pertaining to novel molecular mechanisms there have been tremendous research activities across the world. Citing one such innovative research approach to tackle H. pylori at the genome level is that of researchers at Monash University, Australia who have worked to reverse (U-turn) the evolution of H. pylori traits harmful to humans, such as antimicrobial resistance. Reverse evolution is the reacquisition of a recently lost ancestral state. The researchers are proving that repeated re-introducing of ancestral alleles by horizontal gene transfer (HGT) could make reverse evolution more feasible. The Monash University researchers have worked on evolving populations of an antibiotic-resistant strain of Helicobacter pylori in growth conditions without antibiotics while introducing an ancestral antibiotic-sensitive allele by HGT. Their results show the conditions that could promote reverse evolution in populations of microbes like H.pylori, despite the relatively low spontaneous mutation rates to the reverse allele.
While several researchers are exploring the molecular mechanisms of increasing treatment efficacy, researchers in the bioengineering field are exploring anti-bacterial compounds that can be used instead of antibiotics. For example, researchers from Portugal are exploring bioengineered strategies, namely biomaterials in the form of micro/nanoparticles (MP/NP) for gastric infection management either as (i) H. pylori binders: to bind the bacteria in the stomach and remove it by the gastrointestinal tract; (ii) contact killing agents: to destroy H. pylori in situ; and (iii) drug delivery systems: to transport and release antimicrobial compounds at the infection site.
These MP/NP were prepared using different biomaterials, such as natural polymers (chitosan alone or combined with heparin, alginate or gelatin), lipids (phospholipids, cholesterol), metals (gold, silver) and inorganic compounds (zinc oxide). These biomaterials were selected due to their intrinsic antimicrobial properties, ability to produce pH-responsive delivery systems or to encapsulate lipophilic compounds.
Antimicrobial peptides (MSI-78 and MSI- 78A), plant extracts (berberine, aloe vera, curcumin), bacteria-derived compounds (azurin, rhamnolipids) and polyunsaturated fatty acids (linoleic acid, docosahexaenoic acid), encapsulated or immobilised onto MP/NP, were also studied as alternatives to antibiotics against H. pylori.
Probiotics to combat H. pylori have been well-studied as they display a wide range of antibacterial effects, including the secretion of organic acids and bacteriocins, the formation of H2O2, the inhibition of adhesion processes, and the downregulation of proinflammatory factor expression.
The use of genetically engineered symbiotic lactic acid bacteria as carriers for antimicrobial peptide delivery has also been explored as a treatment option for H. pylori. Also, researchers have that Saccharomyces boulardii and Lactobacillus johnsonni are able to diminish the bacterial load (but do not completely eradicate the H. pylori bacteria when administered as monotherapy).
Emerging techniques to detect H. pylori
Owing to H. pylori’s widespread pathogenesis, rapid and accurate diagnosis of the bacteria and the determination of its antibiotic resistance are important for efficient eradication. The available diagnostic methods can be classified as invasive or non-invasive, and the selection of the best way relies on the clinical condition of the patient, as well as on the sensitivity, specificity, and accessibility of the diagnostic tests.
Currently, H. pylori diagnosis methods mainly include the Urea Breath Test (UBT), the Antigen test, the Serum Antibody Test (SAT), gastroscopy, the Rapid Urease Test (RUT), and bacterial culture. Among them, the first three detection methods are non-invasive, but in these tests bacteria cannot be retrieved through these techniques; thus, drug resistance testing cannot be performed. The last three are invasive examinations, but they are costly, require high skills, and have the potential to cause damage to patients. Therefore, the use of a non-invasive, rapid, and simultaneous method for H. pylori detection and drug resistance testing is of vital importance for efficiently eradicating H. pylori in clinical practice.

Fortunately, the adoption of quantitative polymerase chain reaction (qPCR) for the rapid detection of H. pylori infection and antibiotic resistance is trending today. Unlike bacterial cultures, this method allows for easy, rapid, non-invasive diagnosis of H. pylori infection status and drug resistance. Researchers are increasingly orienting their studies towards quantitative PCR (qPCR) and digital PCR (dPCR) techniques as these offer improved sensitivity and specificity compared to standard PCR, potentially detecting lower bacterial loads, and differentiating active infection from past exposure. qPCR offers a much more accurate way to detect and quantify clinically- relevant organisms than standard PCR, culture, microscopy, or DNA sequencing-based methods. This kind of accurate assessment is essential for helping practitioners to determine the clinical significance of pathogenic organisms like Helicobacter.
Another non-invasive technique that is largely explored is the Faecal Microbiota Analysis (FMA), which analyses the gut microbiome through stool samples. FMA opens doors for non-invasive H. pylori detection along with insights into overall gut health.
In low-resource settings, Point-of-care testing (POCT), i.e., rapid, portable tests utilising various technologies like immunoassays or PCR are being developed for on-site H. pylori detection in clinics or even at home. This could improve accessibility and expedite diagnosis. In many advanced scientific facilities, researchers are collaborating with digital technologists to derive AI algorithms that can be trained using large datasets of clinical data and images to aid in H. pylori diagnosis using existing non-invasive tests. This could improve accuracy and efficiency by identifying subtle patterns missed by human interpretation.
The aforementioned developments are certainly promising, but most of these breakthroughs are still in research or early clinical trials. More validation and large-scale studies are needed before widespread adoption. There exist many gaps in the lab to market solutions for addressing the pathogenesis of H. pylori, which offers tremendous opportunities for budding microbiologists pursuing research.
Could a vaccine solve the problem?
While the search for a vaccine against H. pylori remains active, there is currently no approved vaccine available. The core problem with H. pylori infection is that almost 90% of patients have no symptoms. Owing to this, diagnosing H. pylori infection clinically, is difficult, especially among children, who often have other more common causes of dyspepsia-like conditions. Vaccination against H. pylori, both to prevent and to treat infection, appears to be a better approach to tackling H. pylori.
The argument with regard to administering the vaccine at an appropriate age still remains in most countries. Both prophylactic and therapeutic vaccines are in need. Another side of the argument suggests that H. pylori infection is thought to be the result of the chronic inflammation caused by infection. Hence it is conceivable that a vaccine would not necessarily need to eradicate the infection - but could be an immunomodulatory vaccine that prevents gastritis.
The need of the hour is to work on addressing the factors that hinder the progress of a viable vaccine. H. pylori is known be a master in evading the human immune system, making vaccine design very challenging. Even more challenging is its bacterial diversity, requiring broad-spectrum protection.
One other major reason for the unavailability of a vaccine is the limited funding by major pharmaceutical companies and vaccine manufacturers who haven’t prioritised H. pylori vaccine development despite the extreme global disease burden.
A Call to Action
Well, I started off this article on the intelligent behaviour exhibited by a bacterium, using Helicobacter pylori as an example. The evidence for that behaviour, whether it’s evasion of antibiotics, bypassing the human immune system, or by not showing any symptoms have been explicitly indicated.
We now know that H. pylori-related complications place a substantial burden on healthcare systems worldwide. This results in increased healthcare costs, reduced quality of life for affected individuals, and potential economic losses due to illness and disability - not to forget that H. pylori is becoming increasingly resistant to most antibiotics, making effective treatment more difficult. The lack of a readily available vaccine against H. pylori is a significant concern, considering the widespread nature of the infection and its potential complications.
The call to action is, therefore, to bring about a collective approach to developing a workable solution to H. pylori infection, thereby saving the lives of billions of people.
It is also important to stress the fact that raising awareness about H.pylori, its complications, and the need for effective solutions is crucial. Supporting research efforts and advocating for increased funding, public-private partnerships to bring a collaborative ecosystem are a must, to expedite progress towards better prevention and control strategies at a global level.
Further reading
WHO publishes list of bacteria for which new antibiotics are urgently needed
How to manage Helicobacter pylori infection beyond antibiotics: The bioengineering quest
Are probiotics useful in Helicobacter pylori eradication?
Eight Substances Added to the 15th Report on Carcinogens
Using Probiotics as Supplementation for Helicobacter pylori Antibiotic Therapy
Gastric Cancer Is on the Rise: Screening and Education Are Vital
Helicobacter pylori Vaccine: From Past to Future - Mayo Clinic Proceedings
What Is New in Helicobacter pylori Diagnosis. An Overview
Artificial intelligence-based tools applied to pathological diagnosis of microbiological diseases
Vaccination against Helicobacter pylori – An approach for cancer prevention?
Status of vaccine research and development for Helicobacter pylori
Helicobacter pylori: an up-to-date overview on the virulence and pathogenesis mechanisms







No comments yet