The gut microbiome has been shown to play a supporting role in immunity, metabolism, digestion, and the fight against ‘bad bacteria’ that try to invade our bodies.
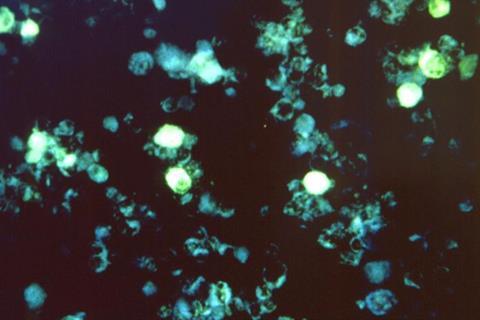
However, new research published in Nature Biotechnology by Angela Wahl, PhD, Balfour Sartor, MD, J. Victor Garcia, PhD, and UNC School of Medicine colleagues has revealed that the microbiome may not as always be protective against human pathogens.
Using a first-of-its-kind precision animal model with no microbiome (germ-free), researchers have shown that the microbiome has a significant impact on the acquisition of Epstein-Barr virus (EBV) and human immunodeficiency virus-1 (HIV) infection and plays a role in the course of disease.
Establishment of infection
“These findings offer the first direct evidence that resident microbiota can have a significant impact on the establishment and pathology of infection by two different human-specific pathogens,” said Wahl, assistant professor in the Division of Infectious Diseases in the UNC Department of Medicine.
This research was conducted through a collaboration with scientists at the UNC International Center for the Advancement of Translational Science and the Division of Gastroenterology and Hepatology at the UNC School of Medicine.
For the discovery to be made, Wahl and Garcia needed to create a ‘humanized’ mouse model that mimicked a human’s immune system to conduct their study. Once exposed to a virus, the humanized models can replicate the virus like a human and could be used for study.
But researchers needed to take it one step further. Wahl and Garcia needed to compare a conventional humanized mouse model to one without a microbiome (germ-free). This meant that they needed to create a first-of-its-kind mouse model that was humanized and free of bacteria.
Humanizing mice
Wahl, who is an expert in developing in-vivo models for human pathogens, and former UNC graduate student Morgan Chateau, PhD needed to figure out a way to humanize the animals while keeping them from encountering any germs, including those that live on our food, skin, in the air, or anywhere else in the external environment.
To do so, they used a custom surgical isolation chamber, which is essentially a ‘big sterile bubble’, with specialized glove compartments and a microscope.
“This had never been done before,” said Wahl, who is also assistant director of the UNC International Center for the Advancement for Translational Science. “We humanized the mice and did our viral exposure experiments under strict germ-free conditions. Technically, it was very challenging.”
Acquiring HIV
HIV is a retrovirus that primarily infects human CD4+ T cells and is mainly acquired through the GI tract. Rectal exposure, for example, in men who have sex with men accounts for more than half of new HIV infections. Breastfeeding is an example of an oral exposure that can also transmit HIV to infants.
Dr. Wenbo Yao, PhD, co-first author, found that rectal HIV acquisition was 200% higher in animals colonized with resident microbiome compared to germ-free animals. Similarly, oral HIV acquisition was 300% higher in animals colonized with resident microbiota compared to germ-free animals. Researchers also noticed that animals colonized with resident microbiota had HIV-RNA levels that were up to 34 times higher in plasma and more than 1,000 times higher in tissues than germ-free mice.
Co-author R. Balfour Sartor, MD, the Margaret and Lorimer W. Midget Distinguished Professor of Medicine in the Division of Gastroenterology and Hepatology and Department of Microbiology and Immunology, says that their innovative HIV findings may ultimately serve as a foundation from which other doctors and researchers can develop new clinical approaches and therapies.
Altering the gut microbiota
“These findings open up a whole new door,” said Sartor, who also directs the National Gnotobiotic Rodent Resource Center that derived the germ- free mice. “Would it be possible to alter the gut microbiota, by decreasing the bacteria, fungi, and viruses that might increase expansion of the HIV infection? Or conversely, we could help patients build up microbes that would prevent that expansion and can work synergistically with antivirals to clear the HIV.”
Researchers then performed comparisons between colonized and germ-free animals, which revealed that the presence of resident microbiota increased the frequency of CCR5+CD4+ T cells, which are the main target of HIV infection throughout the gut. The findings suggest that enhanced HIV acquisition and replication is due to, at least in part, an increased density of target cells for local infection following oral or rectal HIV exposure.
“This is a finding of great significance,” said Wahl. “Everyone has a unique composition of microbes that colonize their gut. In the future, it will be important to evaluate how this diversity among people affects their risk for HIV acquisition and the subsequent course of disease.”
EBV findings
The findings on EBV were also important. EBV is a DNA herpesvirus that infects B cells and can cause mononucleosis. Almost 95% of the adult population harbours latent infection of EBV, but for some people with compromised immune systems, EBV infection can influence the development of certain types of cancers such as Hodgkin’s lymphoma, Non-Hodgkin lymphoma, Burkitt’s lymphoma and Nasopharyngeal carcinoma.
Wahl and Garcia found that mice with a normal microbiome that were exposed to EBV developed large macroscopic tumours in a variety of organs, including the spleen, liver, kidney, and stomach. These tumours were virtually absent in the germ-free mice infected with EBV. Future studies will be needed to evaluate the possible mechanism(s) for enhanced EBV infection and tumorigenesis in the presence of resident microbiota.
“We had two human pathogens that are different in every possible way,” said Garcia, who is director of the UNC International Center for the Advancement of Translational Science, Oliver Smithies Investigator and professor in the Departments of Medicine and Microbiology and Immunology. “The type of cell that they are infecting, the type of disease that they cause, the type of viruses that they are is completely different. Yet, the microbiome exacerbates the disease that each of those viruses causes.”
Pinpointing factors
The researchers will now try to pinpoint the factors that determine whether the microbiome plays a role in the persistence of HIV and EBV infections throughout the body and figure out if the microbiome also affects other human-specific pathogens.
More specifically, Wahl, Garcia, and Sartor want to understand how the microbiome is contributing to HIV and EBV infections. They also want to identify which specific microbial strains are aiding in the viruses’ capacity to replicate and cause disease and of particular importance which ones are protecting the host from the viruses. Sartor, in particular, is interested in learning if there are any additional latent viruses affected by the presence of the microbiome.
“Will the gut microbiome also influence reactivation of herpes simplex virus, shingles, and other latent viruses that cause tumors? We don’t know, but there’s a pretty good track record that microbes can influence certain bacterial and fungal pathogens. That may be something to explore as well.”
Microbiome and pathogens
Wahl and Garcia hope that their findings will open a new area of exploration for researchers who are interested in the role of the microbiome on diseases caused by human specific pathogens.
“At the heart of all this is that we have created a new series of models that will allow investigators to ask questions they could never ask before,” said Garcia. “We have been able to do something that others thought could not be done.”
Other investigators include Joseph Pagano, MD, Craig Fletcher, DVM ,PhD, M. Andrea Azcarate-Peril, PhD, Michael Hudgens, PhD, Allison Rogala, DVM, and Joseph Tucker, MD, PhD from UNC, Christopher Whitehurst, PhD from New York Medical College, and Ian McGowan, MD, PhD from the University of Pittsburgh and Orion Biotechnology.





No comments yet