New research by Adam Rosenthal, PhD, assistant professor in the Department of Microbiology and Immunology at University of North Carolina, has found that some bacteria cells get ’hangry’, releasing harmful toxins into our bodies and making us sick.
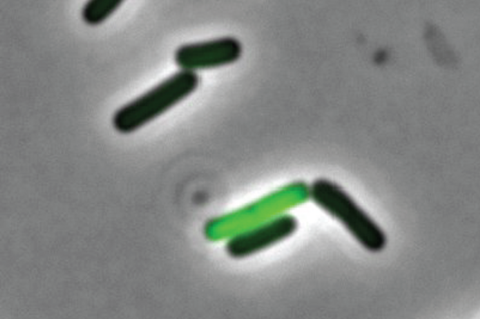
Rosenthal and his colleagues from Harvard, Princeton and Danisco Animal Nutrition discovered, using a recently developed technology, that genetically identical cells within a bacterial community have different functions, with some members behaving more docile and others producing the very toxins that make us feel ill.
“Bacteria behave much more different than we traditionally thought,” said Rosenthal. “Even when we study a community of bacteria that are all genetically identical, they don’t all act the same way. We wanted to find out why.”
The findings, published in Nature Microbiology, are particularly important in understanding how and why bacterial communities defer duties to certain cells – and could lead to new ways to tackle antibiotic tolerance further down the line.
Bad actors
Rosenthal decided to take a closer look into why some cells act as “well-behaved citizens” and others as “bad actors” that are tasked with releasing toxins into the environment. He selected Clostridium perfringens – a rod-shaped bacterium that can be found in the intestinal tract of humans and other vertebrates, insects, and soil – as his microbe of study.
With the help of a device called a microfluidic droplet generator, they were able to separate, or partition, single bacterial cells into droplets to decode every single cell.
They found that the C. perfringens cells that were not producing toxins were well-fed with nutrients. On the other hand, toxin-producing C. perfringens cells appear to be lacking those crucial nutrients.
“If we give more of these nutrients, maybe we can get the toxin-producing cells to behave a little bit better,” postulated Rosenthal.
Researchers then exposed the bad actor cells to a substance called acetate. Their hypothesis rang true. Not only did toxin levels drop across the community, but the number of bad actors reduced as well. But in the aftermath of such astounding results, even more questions are popping up.
Now that they know that nutrients play a significant role in toxicity, Rosenthal wonders if there are particular factors found in the environment that may be ‘turning on’ toxin production in other types of infections, or if this new finding is only true for C. perfringens.
Alternative treatment
Perhaps most importantly, Rosenthal theorizes that introducing nutrients to bacteria could provide a new alternative treatment for animals and humans, alike.
For example, the model organism Clostridium perfringens is a powerful foe in the hen house. As the food industry shifts away from the use of antibiotics, poultry are left defenseless against the rapidly spreading, fatal disease. The recent findings from Rosenthal et al. may give farmers a new tool to reduce pathogenic bacteria without the use of antibiotics.
Rosenthal is in the process of partnering with colleagues across UNC to apply his recent findings to tackle antibiotic tolerance. Antibiotic tolerance occurs when some bacteria are able to dodge the drug target even when the community has not evolved mutations to make all cells resistant to an antibiotic. Such tolerance can result in a less-effective treatment, but the mechanisms controlling tolerance are not well understood.news







No comments yet