University of New South Wales medical researcher Dr David Jacques and his team have discovered how the human immunodeficiency virus (HIV) breaches the cell nucleus to establish infection, a finding that has implications beyond HIV biology.

To infect cells, HIV must enter the target cell and make its way to the nucleus in the cell’s centre where enough copies of its genetic code can be produced to infect other cells.
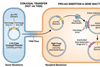
To safely complete this quest, the virus builds a protective protein coat – a capsid – to shield itself from the host’s immune defences geared to destroy it. Until now, it has remained a mystery exactly how the entire capsid moves through the pores embedded in the nuclear envelope to enter the nucleus.
But the research published in Nature today reveals just how the HIV capsid gets into the nuclear pore barrier channel.
Restricted access
“The nuclear pore complex is made up of a combination of proteins,” said the study’s senior author Dr Jacques of UNSW’s School of Biomedical Sciences.
“While small molecules pass in and out of the nucleus via the nuclear pore complex, traffic is restricted for bulky cargo.
“Larger proteins need to be bound to nuclear transporters – chaperone proteins – that carry them through the multi-layered molecular gate.”
Unchaperoned virions
The HIV capsid, despite being a thousand times bigger than the size of molecules that filter through the barrier layers, could pass into the nuclear transport channels without chaperones, Dr Jacques’ team showed.
Chaperone proteins – also called ‘karyopherins’ – engage with the proteins in the middle of the nuclear pore complex in a way that allows them to move with their payload into each successive layer of the molecular gate. Bulky structures without chaperones are excluded from this portal because they are unable to connect with the gate-keeper proteins in the nuclear pore.
Secret handshake
The HIV capsid, however, has evolved to interact with the barrier proteins in the same way as the host’s chaperone proteins.
“One of the theories in the field is that HIV hijacks a host chaperone to gain access to the nucleus. But our results show that HIV does not need a chaperone because it is its own chaperone. It’s as though the viral capsid has learned the secret handshake to be permitted into a restricted area by mimicking the chaperones,” Dr Jacques said.
“People made assumptions about how the capsid might get past the selective barrier. Our work is really starting to directly address this, and to me that’s exciting,” said Dr Claire Dickson, co-first author of the study.
SIngle-molecule method
The discoveries were enabled by a single-molecule method developed previously by the team that allowed them to systematically screen proteins of the nuclear pore complex to identify those that interacted with the intact HIV capsid.
Dr Jacques is particularly excited about bringing together the collective knowhow of his multidisciplinary team in Australia and the UK, and the crucial infrastructure available at UNSW.
“The only way we were able to deliver this project was by tapping into UNSW’s Mark Wainwright Analytical Centre and the expertise that they brought across the breadth of different technologies and methods that we needed, including protein production, structural biology, super-resolution imaging and electron microscopy,” he said.
“I am really proud of being able to overcome some major challenges with this project, to make a significant impact on the field of HIV research by aiding a better understanding of this process,” said co-first author Dr Sophie Hertel.
Wider implications
The molecular understanding gained in this study about host-pathogen interactions, the authors believe, goes beyond uncovering details of the lifecycle of HIV. This mechanistic knowledge can also be exploited for other applications including gene therapy.
“HIV is one of the most studied pathogens, but we still have so much that we can learn from it. There’s something special about HIV; it can penetrate the nucleus without damaging it or needing to wait for the cell to divide like other viruses. Our observations give us insight that allows us to think about how we deliver cargo into the nucleus,” said Dr Jacques.





No comments yet