Viruses in water pose major public health threats, yet their structural diversity makes them unequally susceptibile to disinfection. This study systematically explored how reactive oxygen species (ROS)—including hydroxyl radicals (•OH), singlet oxygen (1O2), and superoxide radicals (•O2⁻)—inactivate viruses with distinct structures.

Using visible-light photocatalysis, the team quantified second-order rate constants and mapped biological damage to viral proteins, genomes, and lipids. The results revealed clear heterogeneity: enveloped RNA viruses were most susceptible to oxidation, while double-stranded DNA viruses showed strong resistance.
These findings uncover the kinetic and mechanistic basis of viral susceptibility to ROS and provide theoretical guidance for advanced oxidation technologies in safe water treatment.
Waterborne viruses
Waterborne viruses such as MS2 and T4 can survive conventional disinfection, posing challenges to public health systems. Advanced oxidation processes (AOPs) generate reactive oxygen species (ROS) that can destroy viral structures, offering promising disinfection solutions. However, the susceptibility of viruses with different genomes and envelopes to specific ROS remains poorly understood.
READ MORE: Gold may be key element for cleaner drinking water
READ MORE: Black gold, blue coke, green solutions?
Previous research has shown that single-stranded RNA viruses are more easily oxidized than DNA viruses, but the kinetics and mechanisms behind these variations are unclear. The complex interactions between viral components—proteins, lipids, and nucleic acids—and ROS still lack systematic characterization. Based on these challenges, it is necessary to conduct in-depth research on the heterogeneous susceptibility of structurally distinct viruses to various ROS.
Researchers from Jilin University and Zhejiang University have uncovered how viruses with distinct structural and genomic features respond differently to oxidative stress. The study (DOI: 10.1016/j.eehl.2025.100178), published on August 20, 2025, in Eco-Environment & Health, demonstrates the kinetic and biological mechanisms underlying virus inactivation by ROS generated through visible-light photocatalysis. Using four bacteriophage models—MS2, phi6, phix174, and T4—the team quantified their susceptibility to hydroxyl radicals, singlet oxygen, and superoxide radicals, revealing key structural determinants that govern oxidative resistance and susceptibility in viruses.
Visible-light catalytic systems
The study employed visible-light catalytic systems using g-C3N4, TiO2, and C60 nanomaterials to generate dominant ROS species (•O2⁻, •OH, and 1O2). Quantitative kinetic modeling showed significant variation in second-order inactivation rate constants, ranging from 105 to 1010 M⁻1 s⁻1.
The viruses exhibited a consistent susceptibility ranking of phi6 > MS2 > phix174 > T4, reflecting their distinct envelopes and genome types. Hydroxyl radicals displayed broad-spectrum oxidative power, while singlet oxygen selectively oxidized capsid proteins, and superoxide radicals preferentially damaged RNA.
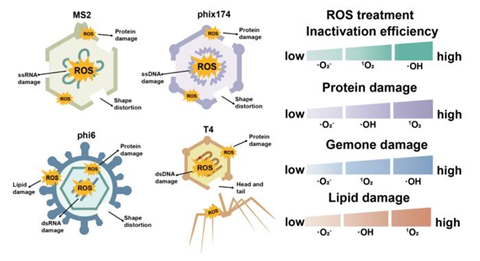
Transmission electron microscopy revealed that ROS exposure caused capsid distortion, head-tail separation, and envelope collapse, depending on the viral structure. Protein assays, nucleic acid degradation measurements, and lipid peroxidation analyses confirmed that the structural complexity of viral proteins and the double-stranded nature of DNA confer greater resistance.
Furthermore, tests in natural water matrices showed that dissolved organic matter and pH significantly reduced inactivation efficiency, with 1O2 proving the most stable and environmentally compatible oxidant.
Targeted disinfection
“Understanding how different ROS interact with viral structures allows us to design more targeted and efficient disinfection systems,” said Professor Cong Lyu, the study’s corresponding author.
“Our results highlight that viral resistance is not random—it’s rooted in molecular architecture. Enveloped and single-stranded RNA viruses are inherently more susceptible to oxidative attack, while complex double-stranded DNA viruses exhibit remarkable resistance. This knowledge provides a scientific foundation for improving AOPs in real-world water treatment, ensuring both safety and sustainability.”
This research offers a mechanistic framework for optimizing water disinfection technologies based on virus type and environmental conditions. By linking viral structure to ROS reactivity, it establishes predictive principles for designing selective and energy-efficient oxidation systems.
The findings suggest that singlet oxygen–dominated photocatalysis, owing to its stability and selectivity, is particularly suitable for complex water environments. Integrating these insights into advanced oxidation technologies could enhance the safety of municipal and wastewater treatment, support emergency epidemic control, and reduce chemical disinfectant usage—advancing sustainable and resilient public health protection strategies.







No comments yet