Some patients with highly drug-resistant tuberculosis could benefit from a shorter treatment with fewer drugs while others may warrant more aggressive therapy, according to the findings of a new study led by an international group of researchers including scientists from Harvard Medical School and conducted across six countries in Asia, Africa, and South America.
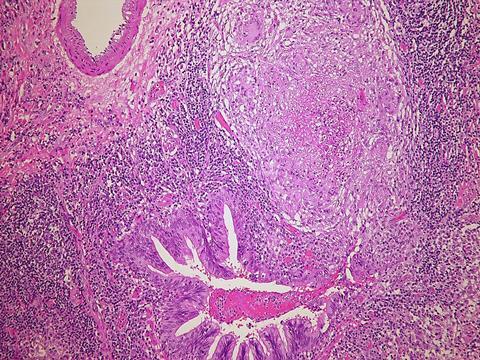
The study, partly funded by the National Institutes of Health, is known as endTB-Q, is the first-ever clinical trial to focus exclusively on people with pre-extensively drug-resistant tuberculosis (pre-XDR-TB), a hard-to-treat form of the disease that is more challenging to cure than multi-drug resistant TB but not as extremely impervious to medicines as the most dreaded form of the infection known as extensively drug-resistant TB. Pre-XDR-TB is resistant to rifampin — the most potent first-line drug used against TB — and fluoroquinolone, which thus far has been the most potent second-line TB drug.
READ MORE: Study finds three new safe, effective ways to treat drug-resistant tuberculosis
The findings, published July 14 in The Lancet Respiratory Medicine, highlight the importance of individualizing therapy to account for patient-to-patient differences and give each infected person a treatment regimen that is the most effective and least toxic for them, the researchers noted.
Tailored approach
“This shorter regimen is not a surefire cure for everyone. The big takeaway is that we might need a more tailored approach to treatment of this kind of resistant TB,” said study TB expert Carole Mitnick, professor of global health and social medicine in the Blavatnik Institute at HMS. Mitnick was co-senior author on the study and a member of the endTB project, spearheaded by Partners In Health, Médecins Sans Frontières, and Interactive Research and Development and done in collaboration with researchers and clinicians worldwide.
In recent years, researchers have increasingly found that shorter, less harsh drug regimens benefit certain patients, Mitnick added, but she cautioned that more research is needed on how to select the right patients who would benefit the most, while ensuring that more severe and more drug-resistant forms of the disease do not go untreated or suboptimally treated, leaving patients with lingering or re-emerging disease that is dangerous to their families and communities.
More than 80 years after the first patients were cured of TB using antibiotics, tuberculosis remains the leading infectious cause of death worldwide, killing close to 1.5 million people a year. The disease has a global reach, including in the United States, where more than 500 people have perished from TB per year for the last decade and cases are on the rise.
Drug-resistant strains
One reason for this is drug-resistant strains of the disease. Another is that many common regimens are difficult for patients to complete, due to the number of pills required, the length of treatment, and the severe side effects of many established therapies. This means that treatment is cut short in some patients, allowing the infection to roar back.
The aim of the endTB-Q trial was to test whether a shorter, likely better tolerated treatment would be effective against pre-XDR-TB. The trial compared an experimental regimen that used a combination of four drugs (bedaquiline, delamanid, clofazimine, and linezolid) for six or nine months with a long regimen based on the standard of care recommended by the World Health Organization, which included four to six drugs taken for 18 to 24 months.
The results of the trial showed that the shorter regimen might be a promising alternative for many patients with pre-XDR-TB. A favorable outcome was established by two consecutive cultures negative for the TB bug late in the 17-month period of post-randomization follow-up or by favorable bacteriological, radiological, and clinical evolution throughout this follow-up. By this standard, the shorter regimen was 87 percent effective while the longer therapy was 89 percent effective. Both groups of patients received social support including access to nutritious food and transportation, shown to help patients complete TB treatment.
Non-inferiority
The research was designed to measure “non-inferiority,” a technical term that describes when an experimental treatment is good enough to replace an existing standard of care. In this study, the shorter regimen did not meet that standard across the full study population.
But not all patients responded the same way to the shorter regimen. Those with more advanced lung damage, for example, did not fare as well as those with less advanced disease. For these individuals, the shorter regimen — even delivered for nine months — was not always sufficient to prevent relapse. These patients benefited more from the longer regimen. This could mean treatment needs to be longer in that group or treatment needs to be reinforced with more drugs, the researchers said.
Mitnick noted that other studies of shortened regimens that have included people with this type of drug-resistant TB in their study population did not have enough statistical power to measure the effectiveness of the regimens on people with pre-XDR-TB or to differentiate between those with different degrees of symptoms.
Guidelines should be updated
The researchers note that recent guidance from WHO and from North American and European experts, which came out after the endTB-Q trial was underway, recommends six-month regimens irrespective of disease severity. Given the findings of the endTB-Q trial and similar results from other studies, the researchers said, the guidelines should be updated to include consideration of stratified approaches to care based on resistance pattern and extent of disease.
“After millennia of fighting this complex, constantly evolving disease, we know that we need to approach it with great caution and attention to detail,” Mitnick said. “Instead of focusing on the ‘prize’ of shortened treatment, we need to keep our eyes on the true goal of curing as many people as we can.”






No comments yet