As the world prepares for future and ongoing global health threats from RNA viruses such as the SARS-CoV-2 pandemic, breakthrough advances in antiviral development are becoming a critical weapon in the fight against these infectious diseases.
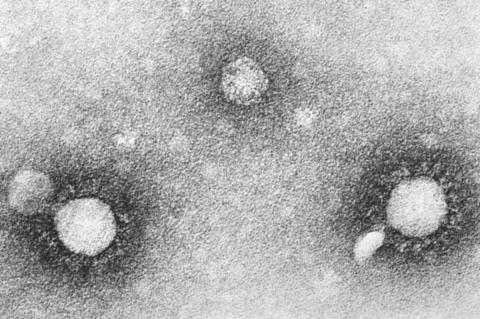
At the heart of this innovation is the exploration of CRISPR/Cas13 systems, which are known for their programmable capabilities to manipulate RNAs and have become indispensable tools for various RNA targeting applications.
However, a significant obstacle has hampered the effectiveness of Cas13d: its restriction to the nucleus of mammalian cells. This drastically limited its utility in cytosolic applications, such as programmable antiviral therapies.
Potent antiviral solution
A scientific team around Prof. Wolfgang Wurst, Dr. Christoph Gruber and Dr. Florian Giesert (Institute of Developmental Genetics at Helmholtz Munich and Chair of Developmental Genetics at TUM), which intensively collaborated with the teams of Dr. Gregor Ebert (Institute of Virology at Helmholtz Munich and at TUM) and of Prof. Andreas Pichlmair (Institute of Virology at TUM), successfully overcame this challenge associated with the cytosolic inactivity of Cas13d.
Through careful screening and optimization, the researchers developed a transformative solution: Cas13d-NCS, a novel system capable of transferring nuclear crRNAs into the cytosol. crRNAs, or CRISPR RNAs, are short RNA molecules that guide the CRISPR-Cas complex to specific target sequences for precise modifications.
In the cytosol, the protein/crRNA complex targets complementary RNAs and degrades them with unprecedented precision. With remarkable efficiency, Cas13d-NCS outperforms its predecessors in degrading mRNA targets and neutralizing self-replicating RNA, including replicating sequences of Venezuelan equine encephalitis (VEE) RNA virus and several variants of SARS-CoV-2, unlocking the full potential of Cas13d as a programmable antiviral-tool.
Redefining the landscape
This important achievement represents a significant step towards combating pandemics and strengthening defenses against future outbreaks. The impact of the study goes beyond traditional antiviral strategies and CRISPR systems and ushers in a new era of precision medicine by enabling the strategic manipulation of subcellular localization of CRISPR-based interventions.
“This breakthrough in antiviral development with Cas13d-NCS marks a pivotal moment in our ongoing battle against RNA viruses,” says Prof. Wolfgang Wurst, coordinator of the study. “This achievement showcases the power of collaborative innovation and human ingenuity in our quest for a healthier and more resilient world.”






No comments yet