Researchers have identified a surprising new player in ALS or motor neurone disease - an ancient, virus-like protein best known for its essential role in enabling placental development.
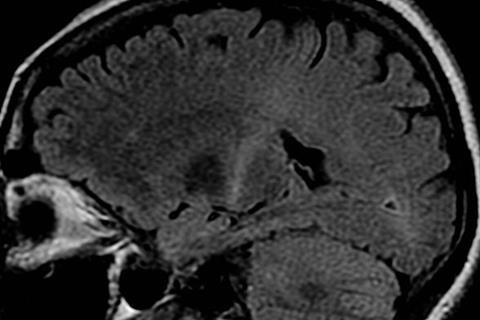
ALS (amyotrophic lateral sclerosis) is a fatal, neurodegenerative disease that attacks nerve cells in the brain and spinal cord, gradually robbing people of the ability to speak, move, eat and breathe. To date, only a handful of drugs exist to moderately slow its progression and there is no cure.
The findings were recently published in the journal eLife.
Strange protein
“Our work suggests that when this strange protein known as PEG10 is present at high levels in nerve tissue, it changes cell behaviour in ways that contribute to ALS,” said senior author Alexandra Whiteley, assistant professor in the Department of Biochemistry, CU Boulder.
With funding from the ALS Association and the National Institutes of Health, and Venture Partners, her lab is now working to understand the molecular pathways involved and to find a way of inhibiting the rogue protein.
“It is early days still, but the hope is this could potentially lead to an entirely new class of potential therapeutics to get at the root cause of this disease.”
Ancient viruses
Mounting research suggests about half the human genome is made up of bits of DNA left behind by viruses (known as retroviruses) and similar virus-like parasites, known as transposons, which infected our primate ancestors 30-50 million years ago. Some, like HIV, are well known for their ability to infect new cells and cause disease.
Others have become domesticated over time, losing their ability to replicate while continuing to pass from generation to generation, shaping human evolution and health.
PEG10, or Paternally Expressed Gene 10, is one such ’domesticated retrotransposon’. Studies show it likely played a key role in enabling mammals to develop placentas—a critical step in human evolution.
But when it’s overly abundant in the wrong places, it may also fuel disease, including certain cancers and another rare neurological disorder called Angelman’s syndrome, studies suggest.
Link to ALS
Whiteley’s research is the first to link the virus-like protein to ALS, showing that PEG10 is present in high levels in the spinal cord tissue of ALS patients where it likely interferes with the machinery enabling brain and nerve cells to communicate.
“It appears that PEG10 accumulation is a hallmark of ALS,” said Whiteley, who has already secured a patent for PEG10 as a biomarker, or way of diagnosing, the disease.
Whiteley did not set out to study ALS, or ancient viruses. Instead, she studies how cells get rid of extra protein, as too much protein has been implicated in other neurodegenerative diseases, including Alzheimer’s and Parkinson’s.
Her lab is one of a half-dozen in the world to study a class of genes called ubiquilins, which serve to keep problem proteins from accumulating in cells.
Faulty gene
In 2011, a study linked a mutation in the ubiquilin-2 gene (UBQLN2) to some cases of familial ALS, which makes up about 10% of ALS cases. The other 90% are sporadic, meaning they are not believed to be inherited. But it has remained unclear how the faulty gene might fuel the deadly disease.
Using laboratory techniques and animal models, Whiteley and colleagues at Harvard Medical School first set out to determine which proteins pile up when the UBQLN2 misfires and fails to put the brakes on. Among thousands of possible proteins, PEG10 topped the list.
Then Whiteley and her colleagues collected the spinal tissue of deceased ALS patients (provided by the medical research foundation Target ALS) and used protein analysis, or proteomics, to see which if any seemed overexpressed. Again, among more than 7,000 possible proteins, PEG10 was in the top five.
Piling up
In a separate experiment, the team found that with the ubiquilin brakes essentially broken, the PEG10 protein piles up and disrupts the development of axons—the cords which carry electrical signals from the brain to the body.
PEG10 was overexpressed in the tissue of individuals with both sporadic and familial ALS, the study found, meaning the virus-like protein may be playing a key role in both.
“The fact that PEG10 is likely contributing to this disease means we may have a new target for treating ALS,” she said. “For a terrible disease in which there are no effective therapeutics that lengthen lifespan more than a couple of months, that could be huge.”
Domesticated virus
The research could also lead to a better understanding of other diseases, which result from protein accumulation as well as keener insight into how ancient viruses influence health.
In this case, Whiteley said, the so-called “domesticated” virus could be rearing its fangs again.
“Domesticated is a relative term, as these virus-like activities may be a driver of neurodegenerative disease,” she said. “And in this case, what is good for the placenta may be bad for neural tissue.”






No comments yet