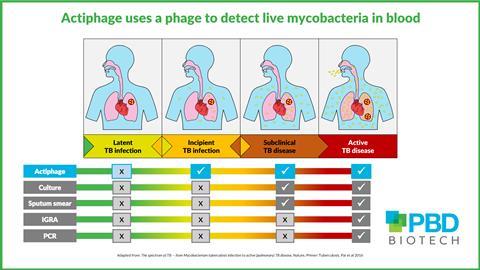
New research has revealed that Mycobacterium tuberculosis – the pathogen that causes tuberculosis – can conceal itself within peripheral blood mononuclear cells (PBMC) to evade detection from the body’s immune system. This knowledge, gained from studies with the phage-based diagnostic Actiphage TB, provides new insights into disease progression.
Now the University of California San Diego (UC San Diego) has donated more than 2,000 previously obtained frozen PBMC samples to accelerate development of this promising diagnostic.
Invaluable support
Jane Theaker, CEO of PBD Biotech, developers of the Actiphage TB blood test, says this support is invaluable.
“Mycobacteria have tough walls and are incredibly resilient and difficult to culture. Phages are the natural enemy of bacteria and provide a mechanism for selectively detecting them in a blood sample, however preparing the samples is far from trivial.
“Our knowledge has so far been gained from fresh whole blood samples; having access to prepared and frozen PBMC reduces the need to recruit more participants and offers another dimension to the study.”
Dr Timothy Rodwell is a Professor at UC San Diego, and a Senior Scientific Advisor at FIND, Switzerland. His research is primarily focused on infectious disease diagnostics with a particular interest in evaluating novel biomarkers for rapid diagnosis of TB.
When he heard about the Actiphage development he was keen to evaluate the technology.
Left-over samples
“There is an urgent need for a diagnostic that can detect those that are at risk of disease progression. We had this set of samples left over from a previous study that were collected, prepared, and stored according to a rigorous protocol. The samples are from those with known infection and their household contacts, so ideal for repurposing in a trial of Actiphage.”
Jane Theaker continues: “We are very grateful to Dr Rodwell and his team. We will be using the samples to look at sensitivity and specificity in index active cases and household contacts, and to follow TB disease progression over time.
“Knowing that the bacteria are still viable after freezing may enable us to use samples from other studies, and this would significantly accelerate the development of new diagnostics.”





No comments yet