Scientists have unraveled the step-by-step activation process of a protein with a deep evolutionary history in all domains of life, opening the door to harnessing its functions for use as a biotechnology tool.

The protein belongs to the ‘superfamily’ of Argonaute proteins, which previous research has suggested to be involved in gene silencing, a fundamental process known as RNA interference.
These proteins are well-characterized in eukaryotes – the plants, fungi, animals, humans and other life forms with cells that have a defined nucleus. In prokaryotes that have no nucleus, there are two types of Argonaute proteins, long Argonautes and short Argonautes. The long Argonautes resemble their relatives in eukaryotes both structurally and functionally. In contrast, short Argonautes adopt different structures and perform different functions from other well-studied Argonautes.
Beginning of blueprint
This is the first study to detail structures and mechanisms of a short Argonaute, potentially sketching the beginnings of a blueprint for application to future therapeutic purposes.
“The short version of these prokaryotic proteins constitute 58% of all Argonautes, and are now emerging as a hot spot in the field,” said senior author Tianmin Fu, assistant professor of biological chemistry and pharmacology in The Ohio State University College of Medicine.
“Among the capabilities we’ve identified is this protein’s precise role in the way bacteria trigger their own death to avoid losing power over their lifecycle through plasmid invasion. Understanding these types of mechanisms is the first step toward efforts to adapt highly effective natural functions for diagnostics and therapies.”
The study is published in Nature.
Plasmid invasion
In this work, the research team focused on a protein called SPARTA, a short prokaryotic Argonaute (also referred to as Ago), specifically building upon other studies that showed this protein enables Maribacter polysiphoniae bacteria to programme their death when they detect a plasmid invasion – when external DNA segments are trying to insert themselves to change bacterial properties.
Ago proteins in eukaryotes are known to remain as simple molecules throughout activation, with the ability to bind only to other simple molecules. They also are established as participants in RNA interference, an evolutionary strategy to inhibit the expression of specific genes that may represent a threat to cell survival.
SPARTA, on the other hand, lacks certain structures that are needed to facilitate RNA interference. And though it starts out as a simple molecule like long prokaryotic and eukaryotic Agos, the activation similarities end there.
Charting next steps
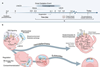
Using cryogenic electron microscopy, researchers identified SPARTA’s next steps: After it binds to RNA or DNA, it goes through numerous changes, eventually assembling into a larger multi-unit molecular complex.
Functional analysis of the complex revealed that the protein’s structural changes had to reach this point before it could produce the chemical reaction that allows threatened bacteria to program their own cell death – an enticing function scientists would like to manipulate to protect human health.
The researchers also introduced mutations to confirm that each step of the process was essential to maintaining SPARTA’s functionality.
All of this points to the fact that oligomerization – the methodical conversion of simple molecules into molecular complexes – is an essential part of activating short prokaryotic Argonaute proteins. While oligomerization of proteins is not rare, understanding its role in a protein’s activation is key to understanding how a protein interacts with other proteins and to determining its functional purpose.
Key protein
“When we talk about one protein that is expressed everywhere, in all organisms, we know this protein is inherently important, even if we don’t yet know all of its specific functions,” said first author Zhangfei Shen, a postdoctoral scholar in Fu’s lab.
“Now that we know not just that it is oligomerized, but how it is oligomerized, and captured the intermediate states it is in during oligomerization, we’ve made good progress toward developing this protein as a tool.”
The possibilities envisioned by Fu’s lab include engineering short prokaryotic Agos that could help cells detect threats, or that could trigger molecules that threaten healthy cells to bring on their own death.
This work was supported by the National Institute of General Medical Sciences.
Additional co-authors include Xiao-Yuan Yang and Kotaro Nakanishi of Ohio State, Shiyu Xia of the California Institute of Technology, and Wei Huang and Derek Taylor of Case Western Reserve University.
Topics
- Argonaute proteins
- Bacteria
- California Institute of Technology
- Case Western Reserve University
- Derek Taylor
- Kotaro Nakanishi
- Microbial Biotechnology
- Ohio State University College of Medicine
- One Health
- Proteomics & Enzymology
- Research News
- RNA interference
- Shiyu Xia
- SPARTA
- Tianmin Fu
- USA & Canada
- Wei Huang
- Xiao-Yuan Yang
- Zhangfei Shen





No comments yet