In the global fight against breast cancer, the power of diagnostic tools can spell the difference between life and death. Survival rates for the disease are greatly improved when the cancer is detected early, while post-treatment detection is paramount to verify the effectiveness of the treatment.

Biomarkers are important in the arsenal of cancer research as they facilitate early detection and can help indicate malignant cells post-treatment to determine if there is any trace of cancer left. Attaining acute sensitivity is therefore crucial. However, the relative amount of cancerous cells in early-stage cases or post-treatment is often minuscule, making their detection challenging.
Associate Professor Desmond Loke from the Singapore University of Technology and Design (SUTD) proposed a novel solution to this problem in a recent paper, ‘Shape complementarity processes for ultrashort-burst sensitive M13–PEG–WS2-powered MCF-7 cancer cell sensors’, published in Nanoscale.
Early stages
“The majority of patients do not exhibit symptoms in the early stages, and the existing diagnostic techniques, which can be inaccurate, costly, and time-consuming, involve imaging tests,” explained Assoc Prof Loke, the project’s principal investigator. “The goal of the research was to create a platform that can identify and treat breast cancer in patients before they show severe symptoms.”
To develop a cell detection system with the highest possible sensitivity, Assoc Prof Loke led a research team—comprising co-workers from SUTD and collaborators from University College London and A*STAR—that used tools at the smallest scale imaginable and worked with nanomaterials. The current technology for cancer cell detection is a digital biomolecular sensor (DBS).
The mechanism works as follows: a chemical recognition element identifies these molecules and converts their interaction into a digital signal that can be easily measured and analysed. This technology is akin to a highly specialised detective tool at the molecular level, with the ability to identify specific biological targets, such as cancer cell proteins, and translate that information into electrical signals that researchers can use for diagnostics research or monitoring.
Low cell count populations
This system, however, is not particularly useful for low cell count populations. The research team hypothesised a newly designed system that would yield higher sensitivity with enhancement from a highly conductive nanomaterial with a strand of viral phage that interact with specific cancer cells.
Enhancing the system required a new 2D nanomaterial with enough electrical conductivity to strongly interact with cancer cell types. The researchers decided upon tungsten disulfide for its high conductivity and use in phototransistors and photothermal therapy. They equipped sheets of tungsten disulfide with a phage-combined polymer that acted as a recognition element for the breast cancer cell types being tested. Integrating the viral agent, or the phage-combined polymer, to the nanomaterial created a new system called phage-based DBS (P-DBS).
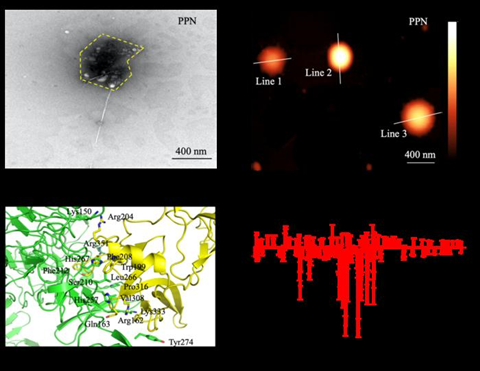
“For the P-DBS technology, when a virus is added to the breast cancer cell sample, the proteins of the virus can show a high specificity for assembly on breast cancer cells. However, it is possible that the virus protein exhibits a specificity that is high enough to assemble on breast cancer cells for a very small number of cells, resulting in ultra-high sensing accuracy,” said Assoc Prof Loke.
Breast cancer cells
Breast cancer cells were studied for this project because viral proteins readily assemble on their surface, allowing a smoother liaison between the P-DBS biosensor platform and the sample cells. According to Assoc Prof Loke, this shape complementarity effect allows for “ultra-accurate sampling, which is essential for early cancer detection and the monitoring of disease progression.”
Four criteria must be satisfied to consider a biosensor highly effective in a clinical context. The biosensor must (1) be highly sensitive to the presence of cancer cell proteins, (2) produce marked contrast in output signals, (3) ensure high cell viability, and (4) produce results within the short reading time common in clinical applications.
P-DBS checked all of the boxes, with reasonable sensitivity. The new technique was able to identify cancer cell presence in samples that were roughly 74% smaller than the typical cell group sizes of other electrical-based cancer cell sensors. The P-DBS also outperformed other electrical-based cancer sensors in terms of signal contrast by 58%. These impressive results can be attributed to the specificity of the viral protein, which the researchers demonstrated would assemble on even the smallest number of breast cancer cells and therefore indicate the presence of cancer even in its early stages.
Significant advance
“The creation of the virus-driven 2D material sensor platform could represent a significant advancement in the battle against breast cancer. If the findings are confirmed in future clinical studies, this sensor might become a valuable accurate tool for identifying breast cancer in its early stages,” added Assoc Prof Loke.
Through further research, he hopes to confirm that the P-DBS system is widely applicable across different breast cancer cell types. The innovative biosensor platform could be significant for early cancer diagnosis and monitoring, demonstrating a promising avenue in the realm of nanoscale biomolecular sensors.






No comments yet