Phages are more than just the latest buzzword, as Tom Ireland reveals - in fact their popularity has waxed and waned numerous times in the decades since the microscopic viral predators were first discovered.
His latest book The Good Virus reveals the roller coaster history of bacteriophages - and why these prolific entities could offer fresh hope to science in a time of surging antimicrobial resistance.

Tom Ireland struggles to pick just one favourite type of phage - and given that these tiny viruses are the most abundant life forms on Earth, that’s not really surprising.
Classic phage
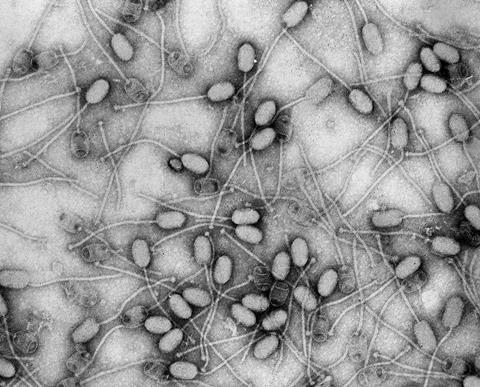
“I guess the T4 phage is the ‘classic’ phage. It’s sinister and quite frightening looking,” he says.
“It’s got this amazing molecular syringe on the end of it that collapses and injects viral genes into the bacteria. Most people haven’t heard of phages but actually, if you show them the T4, they go ‘oh, that’s a virus’, so it’s become sort of a symbol of all viruses.
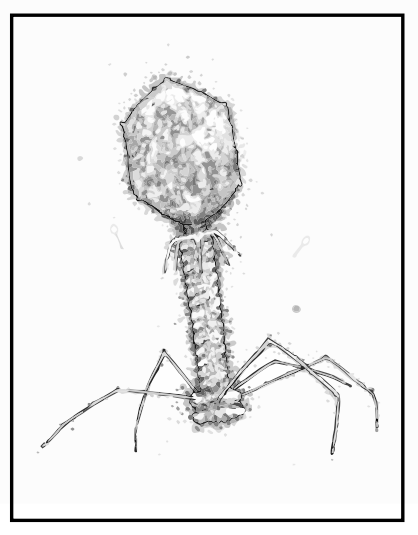
“I think it’s quite cool when you see it on tattoos and posters and band T-shirts and weird places. The T4 is kind of like the rock star of the virus world.”
Unfathomable numbers
But he’s torn between that and the less-than-catchy HTVC01OP - “It definitely needs a better name, but it’s probably the most abundant biological entity on the planet.”
This particular phage stands out because it infects the most dominant marine bacteria in the seas, Pelagibacter ubique, which can be found throughout the world’s oceans..
“There are an unfathomable amount of these viruses in the seas,” he says.
“I’ve always thought it interesting that if an alien were to come and take a random bit of seawater from Earth and look for life, more likely than not they would just find lots of this virus.”
Story of phages
Originally from Poole in Dorset and now living in Hertfordshire with his wife and daughter, Tom has the perfect qualifications to bring the story of phages to vivid life, as the editor of the Royal Society of Biology’s magazine The Biologist for the past 10 years. During that time he had become intrigued by the story of phages and the vivid personalities who have championed them down the years.

“I’d always thought they were really interesting, just as biological entities. The look of them is quite unique, and compared to other viruses they’re a lot more sophisticated. They have these amazing leg-like structures that make them look like nanobots, or tiny spiders,” he says.
“So I’d always thought phages were cool, and I’d heard about them being used as alternatives to antibiotics in the former Soviet Union.
Tangled history
“My original idea was to do a ‘little book of phages’, a short book about the main types of phages and why they are interesting. But when I started to look into the field of phage science deeply, I realised there’s this amazing tangled history, full of eccentric characters who were fighting against a tide of either indifference or outright hostility to their ideas.
“Then it became this quite big book, not just about the exciting possibilities of phages, but how this strange history explains why we are where we are now - with some parts of the world using phages in medicine, but others very much behind and now trying to catch up.”
With the current rise in antimicrobial resistance, there’s a distinct buzz in the West around the potential of phages to treat bacterial pathogens. But that buzz echoes a virtually forgotten period in the 1920s and 1930s when, as Tom puts it, the whole world went mad for phage therapy.
Buzz years
“Phages were being produced in the UK, in the US, in Germany, they were being used in India, Brazil,” he says.
“They were seen as a really promising kind of way of treating bacterial infections, because this was a couple of decades before penicillin was widely available. But essentially, they were quite temperamental, as you had to find exactly the right phage for the particular strain of bacteria that each patient had, and scientists didn’t understand their life cycle very well.
“This was before clinical trials and drug regulation, so companies were soon making broths with lots of different phages thrown in and saying they could treat all sorts of different diseases - and phages developed a bad reputation that they struggled to shake off.
“Then penicillin came along and it was just so much more convenient and consistent for doctors to use, and it treated a broad range of bacteria, rather than having to find phages for every different bacterial strain.”
Soviet surge
It was a different situation in the Soviet Union, where the supply of antibiotics was much less consistent, which is why the Soviet world bought into the potential of phages at the same time as phages were falling out of favour in the West..
By the 1960s and 1970s, phages were being produced on an industrial scale and shipped across the Soviet Union.
Fascinatingly, the public scepticism towards Felix D’Herelle, one of the discoverers of phages, may also have played a key part in the West’s lack of momentum, Tom says.
Having written a number of articles on phages, it was when Tom came across the story of Felix D’Herelle and other phage trailblazers that the subject truly came to life.
Colourful character
“Félix d’Hérelle is just an unbelievable character, really unconventional - self taught, a bit of a blagger, had a reputation for exaggerating his results,” he explains.

“But this was in that era in the 1910s and 1920s when microbiologists were really famous people - Louis Pasteur, Koch, all those people were kind of superstars. And then Félix d’Hérelle came along and said, I’ve discovered a microbe that attacks other microbes that we can use to kill off bacteria.
“And all of these Nobel Prize winning microbiologists just could not believe that this guy had done this with his little lab and with his made-up medical qualifications. Some scientists dedicated years of their life to proving that he was wrong and coming up with alternative theories for what he’d seen when he inoculated bacteria with these bacterial viruses and it killed them.”
Same phenomenon
Those microbiologists then came across a paper from an English scientist called Frederick Twort who had come across the same phenomenon independently a couple of years ahead of D’Herelle.
“So then they started accusing [D’Herelle] of being a plagiarist as well, and essentially trashed his reputation, and that’s why he ended up planning to move to Soviet Georgia, because he was just fed up with being denigrated by by all the establishment microbiologists.

“I just thought he was such an incredible character, and achieved so much with really quite rudimentary equipment and theory. He essentially worked out what was going on way before electron microscopes allowed us to actually see a phage, with really elegant theory and experimentation.”
Lure of Georgia
D’Herelle decided to move to Georgia, where he planned to set up an institute with his friend, the Georgian microbiologist George Eliava, for studying phages and for turning phage therapy into a mainstream form of medicine.
“George Eliava ended up being murdered in Stalin’s purges and that put D’Herelle off from moving to the USSR, which was becoming a very scary place around that time,” says Tom. “But the seed of the idea had taken off in Georgia, and this institute became the pharmacy of the Soviet Union.

“By the 80s they were producing thousands of litres of phage that were being shipped all across the Soviet Union. While in the West, we had forgotten all about phage therapy - and because of the Iron Curtain and this divide between eastern and western scientists, we just didn’t know anything about what was going on in the USSR.
“In Tbilisi, the capital of Georgia now, you can still walk into a pharmacy and get a vial of phages if you’ve got an upset stomach, you can get creams with phages in if you’ve got abscesses or spots that have become infected. And they still have a couple of clinics in the city centre where you can essentially go and get more intensive phage therapy for these conditions like infections where the bacteria are resistant to every antibiotic we have.
Vial of virus
“They really don’t think of it as anything out of the ordinary to drink a big dose of concentrated viruses. It’s an antibiotic that they’ve been taking for decades.
“So in the West, we now have this crisis of antibiotic resistance that’s becoming really acute at the moment and lots of people are thinking, why are we not using phage therapy?”

Even the resurgence of phages in the West is nothing new - they came back on the radar back in the 90s when the USSR collapsed and western scientists learned how they were being utilised in the Soviet world.
“Some people got really excited about it, but it was too weird and too different from how our health systems work to really take off,” Tom says.
Waxing and waning
“Interest has waxed and waned in the idea as people see its potential but then get bogged down in the difficulties of regulation. Using a virus is very different to taking an antibiotic because there’s lots of lab work involved for every patient. You’re also injecting something that’s self-replicating, so getting the dose right is very difficult.
“Regulators like to see trials involving lots and lots of patients, but with phage therapy, it’s kind of bespoke for every patient, and it’s hard to patent treatments because they’re naturally occurring things.
“So there are all these challenges that have kept phage therapy from taking off over the last 20 years, but I think antimicrobial resistance is so worrying now - really it’s not a problem for the future anymore, it’s here and there are millions of people dying now each year from infections that can’t be treated with antibiotics.
“So that has provided a real impetus and I think phages are getting more publicity now, and there’s a big body of evidence now from compassionate use cases that’s really convincing and it’s showing people that this is something we should be taking seriously.”
Last gasp therapy
Even in Western countries that lack a regulatory framework for phage therapy, it can be used on a very limited basis.
“Where someone has basically no options left, regulators will say, ok, you can try phages even though they’re not licensed for use. And that has been a way of probably getting a couple of hundred really sick people phage therapy in the West recently, and the results have been pretty promising, considering that these patients are often really in a bad way by this point.
“There are also lots of clinical trials happening now that have been specially modified to work with the particular characteristics of phages and phage therapy, and recognising that it’s different to treating people with a well characterised chemical.”
Beyond medicine
While the spotlight has focused on phages mainly because of their potential to target pathogens, they offer a host of non-medical applications as well.
“In the book I take readers through the 1950s and 60s where phages were really central to the emerging science of molecular biology. Molecular biologists didn’t really have any tools to study DNA and proteins until Max Delbrook came along and started using phages as a kind of experimental tool - they’re so simple that he was able to study replication in its most fundamental form,” Tom says.
“Things like CRISPR - gene editing - we think of it as a technology that’s been developed by scientists, but actually it’s something that’s evolved in bacteria to protect themselves from phages.
Reprogramming phages
“And then there are all these other ways that phages could potentially be used. They’re essentially little nanoscopic syringes that have evolved to inject their DNA into bacterial cells, but they can quite easily be reprogrammed to target tumour cells, for example, and instead of injecting their own genes, they inject some kind of anti cancer compounds. So there’s some really exciting new ideas for nanomedicines that are phage-based.
“It’s only in the last 30-odd years that scientists have started to look in the environment for phages and think about what role they have in ecosystems and they’ve realised that they’re the most abundant biological entity on the whole planet, that there’s billions of them in every litre of seawater on on Earth.
“All of those phages are mostly unknown to science and they’ve not been accounted for in models of how microbial ecosystems work and how nutrient cycling works. Biologists refer to phages as the kind of dark matter of biology because they’re influencing bacterial populations everywhere, in every possible niche - and we really know nothing about them.”
Phages of the future
Asked to gaze into the crystal ball and gauge what could be coming down the track in future decades, Tom points to some groundbreaking work in Belgium which has become a hotbed for phage therapy because of the slightly looser regulations.
“There are people there who are essentially trying to genetically engineer phages so that they’re much easier to use as medicines, and perhaps even synthesise phages,” he explains.
“So rather than this quite laborious process of taking a bacterial sample from a patient, and then going out to the phage community and saying ‘Does anyone have a phage that can kill this bacteria?’ you use AI and predictive technologies to work out the ideal genome of a phage that would kill this bacteria and you synthesise it there and then by the patient’s bedside.
Sci fi to reality
“So that’s a bit like the science fiction version of phage therapy at the moment, but all of the technology to make that happen does exist. It’s just a case of putting it all together.
“That’s a really exciting avenue, I think, and might help make phage therapy easier to administer and overcome all of these kinds of logistical and regulatory hurdles that have hampered it in recent years.”
Most excitingly for Tom - and for his daughter who was born during the height of the pandemic - he has discovered and named his own phage, as have many other people involved in phage-hunting initiatives.
Pond dipping
“There’s one story of a 10-year-old boy who went dipping in his local stream, and his dad took the water sample to a laboratory to check for phages. And they found a phage that can kill the bacteria that’s classified as the number one most dangerous bacteria on the planet by the World Health Organisation,” he says.
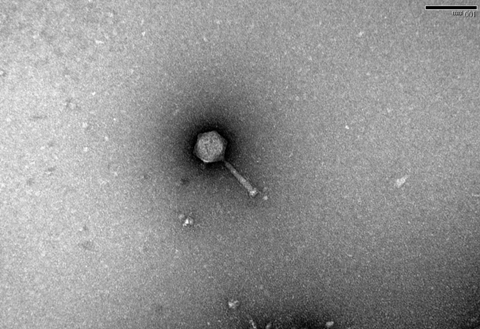
“So I went out and took some samples in my local stream, and the scientists at the University of Exeter filtered the water and washed it over plates of bacteria to see if any bacteria start to die - if they die, then you’ve found a phage.
“I got to name it after my daughter, so I have a phage out there called Emmie23 - so I guess that’s probably my favourite phage!”
The Good Virus by Tom Ireland is published by Hodder & Stoughton RRP £25.
Supporting documents
Click link to download and view these filesEmmiPhage
Image, FileSizeText 6.73 mb




No comments yet