Norovirus is a leading cause of acute viral gastroenteritis worldwide with severe outcomes mostly among young children, the elderly and people with weakened or compromised immune systems. There are currently no approved vaccines or antiviral therapies, and management strategies rely solely on supportive care, including fluid and electrolyte replacement.
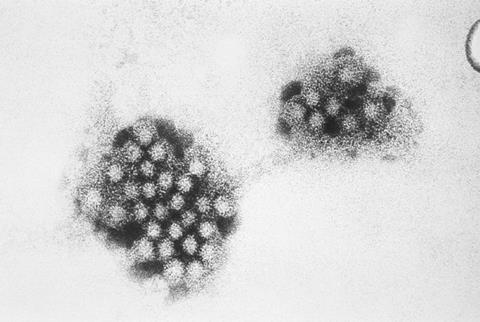
Researchers at Baylor College of Medicine have reported a breakthrough in human norovirus (HuNoV) research in Science Advances. In the current study, the Baylor team has overcome a major obstacle that limited their ability to continuously grow virus, which they require to conduct experiments needed to develop strategies to prevent and treat infections and better understand HuNoV biology. The researchers identified factors that restrict viral replication and developed a way to overcome them to optimize long-term viral cultivation.
READ MORE: Some human GII.4 norovirus are better than others at infecting cells; researchers have found out why
READ MORE: Researchers discovered replication hubs for human norovirus
“In 2016, a previous breakthrough occurred when scientists in our lab and collaborators successfully grew HuNoV in human intestinal enteroids (HIEs), or ‘mini-guts’– miniature, lab grown versions of the human gut,” said first author Gurpreet Kaur, graduate student in molecular virology and microbiology at Baylor working in Dr. Mary Estes’ lab.
“While this system allowed researchers to infect cells and study the virus, it still had a major shortcoming – the virus would not grow through repeated rounds, the way scientists can grow many other microorganisms. After just a few rounds, norovirus replication would stop, making it impossible to build up long lasting viral stocks.”
Depending on stool samples
Because of this limitation, researchers have depended on virus collected from infected patients’ stool samples, which are limited, inconsistent and make large scale experiments difficult.
“Looking to overcome this obstacle, we studied several versions of HIEs to understand why norovirus growth usually stops,” said co-author Dr. Sue Crawford, assistant professor of molecular virology and microbiology at Baylor. “Using RNA sequencing, a method that measures gene activity, we discovered that infected HIEs produced high levels of chemokines, molecules that help the body mount an immune response. Three chemokines stood out: CXCL10, CXCL11 and CCL5.”
“We then investigated whether blocking signaling of these chemokines through their receptors would allow human norovirus to replicate better in HIEs,” Kaur said. “We tested a drug called TAK 779, originally developed to block chemokine effects. When TAK 779 was added to the HIE cultures, norovirus replication increased dramatically – virus spread throughout the cells in the cultures, and we achieved replication for 10 to 15 consecutive passages.”
“TAK 779 allowed us to generate, consistent batches of infectious virus from lab cultures instead of human stool — something we and other researchers have been seeking for decades,” Crawford said.
Not all the same
The team also learned that not all HuNoV strains respond to TAK 779 the same way. TAK-779 enhanced replication of strain GII.3, and the growth of strains GII.17 and GI.1.
“We observed that TAK 779 did not enhance replication of GII.4 strains, the most common cause of human outbreaks,” said corresponding author Dr. Mary K. Estes, Distinguished Service Professor and Cullen Foundation Endowed Chair of molecular virology and microbiology at Baylor. Estes also is the co-director of the Gastrointestinal Experimental Model Systems core at the Texas Medical Center Digestive Diseases Center and a member of Baylor’s Dan L Duncan Comprehensive Cancer Center.
“This difference appears to be because GII.4 viruses do not trigger chemokine secretion in HIEs, meaning there’s no chemokine response for TAK 779 to block. This suggests that a different process limits GII.4 growth in HIEs. We are currently optimizing our HIE culture conditions to enable efficient passaging of additional HuNoV strains, including GII.4.”
Leap forward
This work represents a major leap forward for norovirus research. By continuously growing and maintaining norovirus strains in the lab and producing stable virus stocks for experiments, researchers can conduct comprehensive studies of viral structure, antiviral drug screening and vaccine development, even in labs without access to patient stool samples.





No comments yet