Thousands of lab animals are used every year to test wound treatments - from mice and guinea pigs to rabbits and pigs.
But one lab in Wales is pointing the way towards a more humane model of research, with the work it is carrying out on in vitro/animal replacement models.
Dr Sarah Hooper, Reader in Microbiology and Infection at Cardiff Metropolitan University, is now onto the third iteration of a pioneering model that can mimic chronic wound infections and investigate biofilms while reducing animal use.
READ MORE: Your Pseudomonas aeruginosa biofilm may vary - depending on where it turns up
READ MORE: Current standards for testing wound dressings don’t work for biofilms, study finds
“We designed, optimised, and validated a novel, reusable biofilm flow model in 2018, and made it freely available so others could benefit. The entire system can be 3D printed for around £200, and we published an updated version in 2023. That work is continuing now with funding to develop a third iteration,” she says.
“We created the model because commercial biofilm systems are expensive, hard to modify, and often not designed to replicate the chronic wound environment.
“Our aim was to create an affordable, open-source, and sustainable platform that other researchers, including those in resource-limited settings, could use. All design files and protocols are freely available online to support transparency and reproducibility.”
Lab animals
Thousands of animals are used every year to test wound treatments including pigs, rabbits, guinea pigs, and mice, Dr Hooper says.
“These models involve biopsy, burn, or incision, with wounds held open to mimic chronicity. But they don’t biologically replicate human wound infection well,” she explains.
“At the same time, the translation of antimicrobial dressings from animal models into the clinic remains poor. Something isn’t working.”
Three goals
Dr Hooper, a member of AMI’s One Health Scientific Advisory Group, said the intention was to devise a model that would address three things:
- Reduce animal use
- More accurately model chronic wound infections, so that bacterial behaviour and antimicrobial response match real-world infections.
- Offer a low-cost, reusable, and open-access platform to democratise research, supporting reproducibility and wider use.
Using CAD software, the team developed a biofilm flow device capable of growing up to 12 biofilms at once under continuous flow. It supports both single-species and polymicrobial cultures (up to five species) and can maintain biofilms in steady state for extended periods.
Chronic wound biopsies
The researchers have discovered that the spatial distribution of bacteria in the model mirrors what’s seen in chronic wound biopsies, an important validation of biological relevance. They have also found that common wound dressings behave the same way in their system as they do in patients, giving confidence that it is fit for real-world testing.
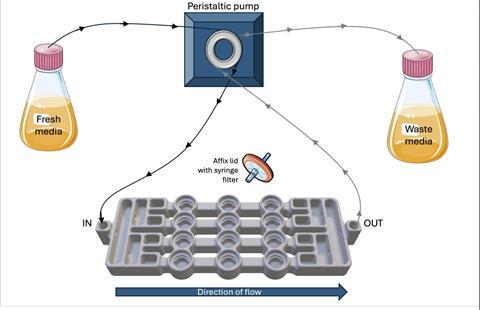
“I’m still surprised by how well something we designed ourselves actually works! One major finding was how much biofilm composition influences antimicrobial efficacy. This difference was far greater than we expected compared to standard lab testing,” Dr Hooper says.
“I’ve also been pleasantly surprised at how keen industry partners are to work with a realistic, affordable model. Several manufacturers have asked us to help test prototype wound antimicrobials because they want robust, clinically relevant challenge data.”
Refining the model
The most relevant paper was published in 2023, but the team is in the process of refining the model further and work is due to finish in December.
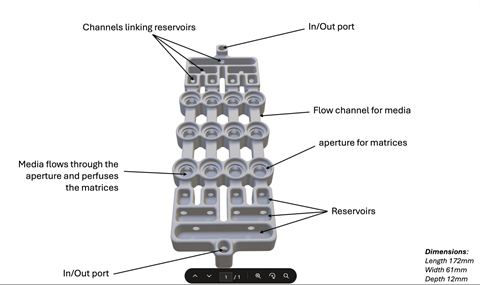
“This work matters because we’re addressing three problems at once: overuse of animal models that don’t translate well, lack of reproducibility and accessibility in biofilm testing, and poor real-world performance of many wound therapies,” Dr Hooper says.
“If we can properly model chronic infection and assess antimicrobial efficacy more realistically, we improve the chances of new treatments working when they reach the clinic, potentially transforming outcomes for patients with wounds that last months or even years.”
More to come
The first two versions of the model were developed by PhD students Peter Duckworth and Ammara Khalid and their publications have helped to secure funding from Replacing Animal Research to keep the project running.
“Thanks to funding from RAR, we’re now working on an improved third version of the platform and look forward to sharing it with the research community soon,” Dr Hooper says.
To find out more, visit Hooper Group: Biofilm Models.
Topics
- Ammara Khalid
- Applied Microbiology International
- Bacteria
- biofilm flow device
- Biofilms
- Cardiff Metropolitan University
- chronic wound infections
- Community
- humane research
- Infection Prevention & Control
- Innovation News
- lab animal replacement
- Microbiological Methods
- One Health
- Peter Duckworth
- Replacing Animal Research
- Sarah Hooper
- UK & Rest of Europe






No comments yet