A newly developed intranasal vaccine candidate helps to clear COVID-19 infections more quickly than controls in pre-clinical testing, according to a new study.
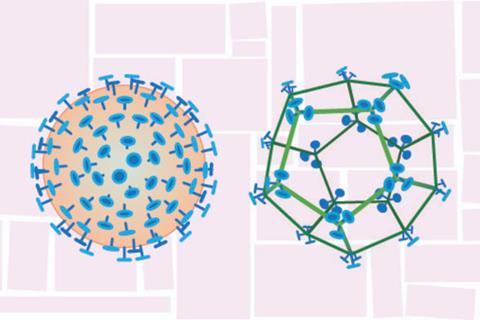
The new vaccine platform relies on a protein scaffold that resembles a tiny wire-frame soccer ball, roughly the size and shape of a virus. When the surface of the scaffold is decorated with a portion of the spike protein from the SARS-CoV-2 virus — the same protein used in existing COVID-19 vaccines — and delivered via the nose, it induces an immune response in a rodent model.
A paper describing the study, led by researchers in the Huck Institutes of the Life Sciences at Penn State, published recently in the journal Microbiology Spectrum. The study demonstrates proof-of-principle for the scaffold, called “SpyCage,” as a potential new delivery system for safe and effective nasal vaccines. While further study is needed to optimize the system to achieve full protection, the SpyCage platform could be easily adapted for use against a variety of respiratory viruses, according to the research team.
Mucus membranes
“Vaccination is a safe and effective method to reduce the impact of harmful infectious diseases and has been crucial to slowing the COVID-19 pandemic,” said Scott Lindner, associate professor of biochemistry and molecular biology in the Eberly College of Science at Penn State and one of the leaders of the research team. “To date, essentially all COVID-19 vaccines are delivered by an injection into muscle. While effective at reducing the severity of infection and associated symptoms, these vaccines do not completely prevent infection or transmission of the virus to others.”
According to the researchers, intramuscular vaccines induce a systemic immune response in the body, but do not induce a strong immune response in mucus membranes, like in the nose and airways. Protection in the body’s mucus membranes, where the virus is first encountered, is essential to preventing infection and limiting transmission of respiratory viruses.
“An effective vaccine administered via the nose has the potential to induce a mucosal immune response as well as a systemic response,” said Troy Sutton, assistant professor of veterinary and biomedical sciences at Penn State and a leader of the research team. “We are developing and testing the SpyCage vaccine delivery system to try to create vaccines that do just that. If we can induce a robust immune response in the respiratory tract, thereby blocking infection and transmission, we may be able to slow the spread of airborne viruses and potentially end, or even prevent, a pandemic.”
Virus-like nanoparticle
According to the researchers, candidate nasal vaccines that have been developed to date have used viral vectors or live-attenuated viruses and continue to have safety concerns, especially for the most vulnerable populations.
“The SpyCage protein scaffold looks similar enough to a virus to trick the immune system into mounting a response, but none of it is actually derived from a virus,” Lindner said. “We make portions of the SARS-CoV-2 spike protein in the lab and permanently attach them to the surface of the SpyCage. The result is a nanoparticle that resembles the virus in size, shape and symmetry that can induce an immune response in our pre-clinical animal testing.”
In their tests, when the SpyCage was loaded with the spike protein and administered via the nose, it induced an immune response helping to clear an infection more quickly than in unvaccinated animals or if the spike protein was administered on its own. If the two components — the SpyCage scaffold and the spike proteins — were administered together but not bound to each other, the researchers did not see an immune response or reduction of infection. They also tested the vaccine in conjunction with an adjuvant — a substance designed to enhance the efficacy of nasal vaccines — but did not see any significant improvement.
Spike proteins
“It’s clear that the SpyCage must be decorated with spike proteins to train the body’s immune system, so that when it is exposed to the actual virus, it can quickly respond and more rapidly eliminate the virus,” Lindner said. “The SpyCage vaccine platform must still be optimized to achieve the goal of preventing infections and transmission, and we are working to improve its efficacy.”
The researchers explained that the SpyCage vaccine platform continues to be developed and is the subject of several patents and pending patent applications.
“More testing is required to confirm its safety and optimize its effectiveness,” Sutton said. “However, because of its modular design and assembly, SpyCage can be easily adapted to make vaccines against other respiratory viruses and could provide a platform for rapid response vaccines for novel viruses in the future.”
In addition to Lindner and Sutton, the research team includes Devanshi Patel, Allen Minns, Derek Gordon Sim, Cassandra Field, Abigail Kerr, Talia Heinly, Erin Luley, Randy Rossi, Carol Bator, Ibrahim Moustafa and Susan Hafenstein at Penn State and Elizabeth Norton at Tulane University. Funding from the U.S. National Institute of General Medical Science, Penn State, a Huck Institutes of Life Sciences COVID Seed Grant and the U.S. Department of Agriculture’s National Institute of Food and Agriculture supported the research.






No comments yet