Cryptococcosis, a severe fungal infection causing over 600,000 annual deaths, has limited treatment options with mainstream therapies such as amphotericin B (AmB), fluconazole (FLC), and flucytosine (5-FC) due to their severe toxicity, frequent drug resistance, and high costs.

Consequently, developing novel anti-cryptococcal agents is critically urgent. In this study, led by Prof. Liu, Institute of Microbiology, Chinese Academy of Sciences, three new phenolic bisabolane sesquiterpenoids (PBS) derivatives (±)-aspersydonol A (1a/1b) and aspersydonol B (2), along with 12 known analogues, were isolated from the marine-derived fungus Aspergillus sydowii LF51 using a combination of molecular networking and SMART approaches (Figure 1).
Among them, compound 2 represents the first example of natural PBS with a hexahydrodibenzo[b,d]furan skeleton. Their structures, including absolute configurations, were experimentally elucidated by spectroscopic analysis, NMR analysis, as well as ECD calculations.
Antifungal assays
Antifungal assays revealed that compounds 3, 6, 9, and 11 exhibited moderate activities against Cryptococcus spp. (MICs = 32–64 μg/mL), with additive effects against Cryptococcus gattii R265 combined with Amphotericin B (AmB), reducing MICs to 4–32 μg/mL.

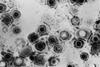
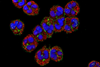
Compound 9 showed an additive effect with fluconazole (FLC) against C. gattii R265, lowering its MIC to 2 μg/mL. Mechanistic studies revealed that compound 9 inhibited C. gattii R265 by suppressing urease activity, disrupting membrane integrity, and inducing oxidative damage via ROS accumulation.
These findings not only expand the chemical space of PBS compounds but also broaden their scope of activity, providing promising lead compounds for the development of anti-Cryptococcus drugs.





No comments yet