An oral lipid nanoparticle drug prevents tumor development in mice, suggesting this is a promising drug formulation for preventing colitis-associated cancer, according to a study led by researchers in the Institute for Biomedical Sciences at Georgia State University.

The findings published in the journal Pharmaceutics report the oral drug formulation consists of M13-loaded nanoliposomes (M13-NL), also known as lipid nanoparticles. M13 is a promising anti-ulcerative colitis compound.
Patients with inflammatory bowel disease (IBD), which includes ulcerative colitis and Crohn’s disease, have an increased risk of developing colitis-associated cancer, which has been found to be unresponsive to standard chemotherapy regimens. Early diagnosis and treatment of colitis-associated cancer can significantly increase patient survival because drug treatments are most effective in the early stages of cancer progression.
Side effects
Current IBD drug treatments have severe side effects, depending on the specific medication used, which can include immunosuppression, bone loss, liver toxicity, pancreatitis and blood disorders. A safe and convenient treatment is needed that can effectively target and release drugs to diseased tissue, reduce side effects and improve symptoms. Current treatments don’t use effective small-molecule drugs and colon-targeted delivery systems, according to the research study.
Previous studies have found the M13-NL formulation can effectively target the colon and reshape gut microbiota in cultures outside of the organism, generating altered microbial metabolites that can efficiently prevent chronic ulcerative colitis.
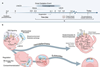
This study tested the cancer cell uptake ability of the lipid nanoparticle formulation and investigated the potential of the M13-NL formulation to prevent colitis-associated cancer in mice.
Lipids assemble
“In prior studies, we demonstrated that lipids extracted from ginger-derived nanoparticles can be assembled to target specific parts of the digestive tract and support the efficient oral delivery of small molecules and siRNAs,” said Dr. Didier Merlin, senior author of the study, Regents’ Professor in the Institute for Biomedical Sciences at Georgia State, and senior research career scientist at the Atlanta Veterans Affairs Medical Center. “The assembled lipid nanoparticles also trigger less toxicity than traditional nanoparticles.”
“In this study, we loaded these lipid nanoparticles with M13, which is a promising anti-ulcerative colitis compound, and used the generated M13-nano-liposome as an oral formulation to treat colitis-associated cancer. Our findings demonstrate that oral administration of M13-NL prevents tumor development in mice, suggesting that M13-NL is a promising candidate for preventing colitis-associated cancer in patients with inflammatory bowel disease.”
Two critical factors
The mice used in this study combine two critical factors involved in colitis-associated cancer development, chronic inflammation and DNA damage. They do not produce the anti-inflammatory cytokine, IL-10, in the colon, so they experience chronic inflammation. The compound azoxymethane (AOM) induces DNA damage in the colon, leading to the development of colorectal tumors. This allowed the researchers to assess the in vivo efficacy of the long-term oral administration of M13-NL against the development of colitis-associated cancer, the study explained.
Further studies are needed to validate these findings and assess the safety and efficacy of M13-NL through human clinical trials, according to the study.
Additional authors of the study were Dingpei Long, a Crohn’s & Colitis Foundation research fellowship award recipient, and Junsik Sung of the Institute for Biomedical Sciences at Georgia State; Zahra Alghoul of the Institute for Biomedical Sciences and Department of Chemistry at Georgia State; and Chunhua Yang, the corresponding author of this study, of the Institute for Biomedical Sciences at Georgia State and the Atlanta Veterans Affairs Medical Center.
The study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health and the Department of Veterans Affairs.





No comments yet