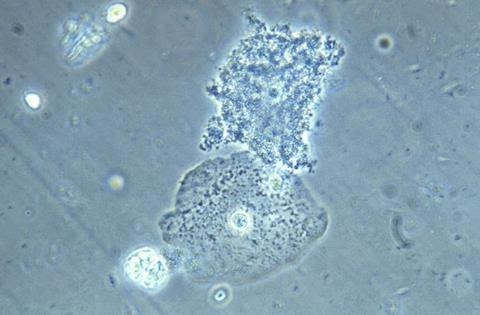
Bacterial Vaginosis (BV) affects about one-quarter of reproductive-age women and is linked to adverse health outcomes, such as increased HIV risk. Yet for decades, BV treatment in the United States has largely relied on antibiotics, and BV recurrence is common following antibiotic therapy.
Now, two European clinical trials have shown limited success with a different type of medication used to treat BV called dequalinium chloride (DQC).
DQC—an antiseptic—has been in use for several decades in countries throughout Europe as an alternative treatment for BV. It is not currently approved by the U.S. Food and Drug Administration.
In a commentary published May 2 in JAMA Network Open, researchers from the Institute for Genome Sciences (IGS) within the University of Maryland School of Medicine (UMSOM) and Johns Hopkins University School of Medicine (JHUSOM) have called for more robust clinical trials in the United States to confirm if DQC is as good or better than existing BV treatments.
Critical need
“For women suffering from BV, there is a critical need for more effective treatments,” said corresponding author Rebecca Brotman, PhD, MPH, a researcher at IGS and UMSOM Professor of Epidemiology and Public Health. “We need more robust clinical trials to fill in the knowledge gaps of what we know about DQC from the European studies.”
In the commentary, the authors discuss three main knowledge gaps from the European trials.
“First, we know that vaginal microbiota may vary regionally and the DQC clinical trials so far have only been conducted in Europe,” said first author Kayla Carter, PhD, MPH, a postdoc in the Brotman Lab at IGS. “In addition, the trials did not last longer than five weeks, so we don’t know long-term outcomes after DQC treatment; and, finally, there’s very limited data on its use and its safety during pregnancy.”
Innovative treatments
DQC works differently than current treatments because it is an antiseptic with antibacterial and antifungal activity, rather than an antibiotic. It also is an intravaginal tablet, not an oral treatment. The antibiotic treatments currently available to U.S. women are metronidazole and clindamycin as first-line medications, with alternatives of secnidazole and tinidazole. While these treatments are generally effective in the short term, as many as 50 percent of women will have a BV recurrence by six months after treatment.
“We’ve seen a growing investment in innovative BV treatments in recent years, including live biotherapeutics and vaginal microbiome transplants, but those are still in relatively early stages of development,” said Dr. Brotman. “In the meantime, the European trials indicate that DQC could be a viable, well-tolerated alternative BV treatment. That’s why it deserves further investigation with well-funded clinical trials.”
Susan Tuddenham, MD, MPH, Associate Professor of Medicine at the Johns Hopkins University School of Medicine also contributed to this commentary.



No comments yet