Six years ago, just as I was finishing up my microbiology PhD, I stumbled across an opportunity to help a patient. One patient turned into many, and what I used to think was 50 years away — using bacteriophages to treat infections — has become my daily work.
I now help set up and manage systems to give patients and their doctors safe access to phages when antibiotics fail. I’m excited to paint a picture of how a microbiology background can translate to helping patients in fewer steps than one might think.
The case for phage therapy, and why it’s hard
Bacterial infections kill millions each year. Antibiotics used to serve us, but after decades of use, bacteria have evolved resistance. We need new solutions.
Phages are bacterial predators. They’ve evolved to precisely target and kill specific bacteria without harming the rest. Phages have been used therapeutically for about a century, but if you don’t choose the right phage for a patient, it doesn’t work. Matching phages with a patient’s unique bacterial strain takes time and infrastructure that clinics don’t have, and getting the funds together to test ‘personalised, living antibiotics’ in clinical trials has been hard for everyone who’s tried.
Because of this, most countries have no phage drugs on the market. This leaves compassionate grounds as the sole option for phage therapy, permitted on a case-by-case basis only when all standard treatments have been exhausted. Often, patients wind up on death’s door before anyone considers phage therapy.
No way but Twitter to find phages for patients
One day in 2017, 25-year-old Mallory Smith was fighting for her life in Pittsburgh; Burkholderia had taken over her lungs due to cystic fibrosis. I was a grad student studying phages at the time, having coffee with my partner Jan when I saw the tweet. It was from Steffanie Strathdee, a patient advocate who’d managed to find phages to save her husband’s life the year prior, asking for phages for Mallory.
Soon I saw someone reply to the tweet — the lab next door to where I did my PhD in Edmonton, Canada. They confirmed they were happy to put the phages in the mail for Mallory. What a small world, I thought. And who knew academic labs could help?
Jan, a software developer, was perplexed. ‘Wait, so doctors need these things, and labs like yours have them in their labs… and the only thing they don’t have is a way of finding each other?’ I nodded, and Jan immediately set about building a website to fix this issue. Maybe we could do it in time to save Mallory.
Setting up a dedicated network to find phages for patients
Over the next few days, we set up ‘Phage Directory’, a website where labs could sign up, list which phages they had, and subscribe to ‘phage alerts’ if a doctor had a patient in need.
A few days in, labs and volunteers started to join our cause. It felt like it was working. But Mallory died 2 days later. We were all saddened and shocked, but it was obvious that the phage research community was ready to help. We kept working on it, reaching out to labs we knew, getting the word out. Eventually, we had our second urgent patient request; this time in France. We sent out our phage alert and connected labs who replied with the doctor who’d requested the phages. It was working! But then that patient died, too.
We kept going. One time there was an alert from Finland that attracted multiple labs — 200 phages were sent to Finland for screening. They even found about 10 phages that worked! But the patient’s situation changed, and the doctor decided not to try phages.
It was a year later when we had our first success. A sea turtle. Finally, everything had lined up. Phages requested, phages tested, phages found! The phages were prepared, the treatment plan was cobbled together. The turtle was treated, and she improved; a years-long shell infection had resolved. So this could work, we realised. We just had to keep going.
Challenges for labs: who owns the phages? And who’s at fault if they fail?
We talked to many research labs in those days. In the beginning, most didn’t realise they could help even if they wanted to. We would tell them how they could share phages and/or receive strains. Many wanted to help, but for several, their universities were concerned. If a patient died, would their university be liable?
Some researchers worked with their universities to come up with contracts protecting against this, and we helped share them with others as templates. We also helped find companies to do the final purification of the phages to ease the burden on academic labs. Later, some labs (such as TAILOR Labs at Baylor College of Medicine) figured out how to meet the FDA’s requirements on endotoxin limits in experimental phage preparations, and offered to help. We would point labs to centres like TAILOR for final purification, meaning labs could now help with just the aspects they were comfortable with (like receiving the strain and screening against their phages) and pass on the phages after that.
Another challenge was the timeframe. Patients eligible for phage therapy have to be so sick they qualify, but not so sick that they can’t wait a few weeks or a month for phages to be sourced and prepared. This still remains a major challenge, though we have found that many patients do meet these criteria, and as long as clinician expectations are set, it can be workable.
First successful phage alert: a kid gets to keep her leg
Finally, in 2019, we had our first human success story. This time the request came from Prof Jon Iredell’s team in Sydney, Australia. Seven-year-old Dhanvi faced a dire situation — highly antibiotic-resistant P. aeruginosa osteomyelitis after a car accident. Facing amputation, her treating physician, Dr Ameneh Khatami, wanted to use phage therapy but lacked a source of phages. The team didn’t think anyone would respond to our alert, given their location, but thought it was worth a try.
To all of our surprise, that phage alert resulted in our largest response yet: 12 labs from 12 countries stepping up within 24 hours. The Sydney team, thrilled by this response, was able to get ethical and legal approval to treat, and everything fell into place.
Six weeks after their call-out, Dhanvi became the first child in Australia to undergo phage therapy. Her infection was eliminated and she was able to avoid amputation.
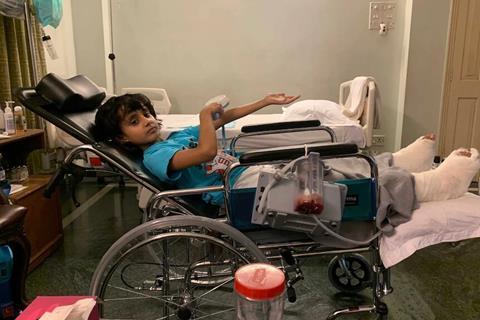
A collaboration with Phage Australia begins
We soon learned of the Iredell team’s plans to scale Dhanvi’s success to more patients, by building an Australia-wide ecosystem for phage therapy. We brainstormed how we might work together to not only treat patients, but to capture the data in a systematic way, building on what they’d already done for a trial testing phage therapy against Staphylococcus aureus in adults. We began working on a grant to get the funds to do it.
A unique clinical trial: the STAMP protocol
Although the pandemic froze our grant plans for a while, the funding came through, and Jan and I moved to Australia in February 2022 to join the Iredell team. By the time we arrived, the team had registered a special clinical trial protocol for phage therapy called STAMP (Standardised Treatment and Monitoring Protocol for Adults and Pediatric Patients).
In a way that hadn’t been done before, the STAMP protocol shifted the focus from testing phage as a product (as you would for a drug) to testing the process of phage therapy. This meant that patients with different infection sources and pathogens could be treated with their own sets of phages, with dosing and monitoring being done systematically, enabling structured sample and data collection from a large and heterogeneous set of patients.
Coming up with a system for phage production
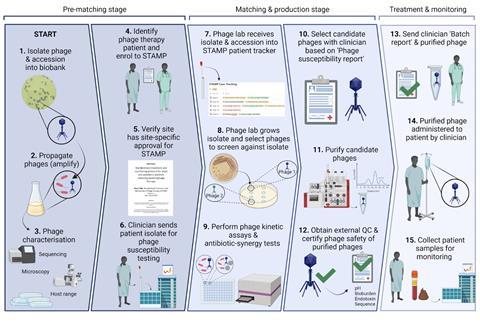
Although the team had gotten the STAMP trial approved, they didn’t have a source of clinical grade phages. But they did have about 30 eligible patients, the strains infecting them, and about 300 phages. We began devising a plan to select, produce and purify phages for the most urgent patient on our list — a man who’d been on antibiotics for 3 years due to an E. coli infection in his chest.
To verify the safety of what we produced, we set up a framework based on the one endorsed by Belgium’s health authority, which involves checking phage genome safety, sterility and endotoxin levels. We then devised a set of reports to detail what we’d done, working around what the clinicians and regulators wanted to know.
Finally, we handed the phages to the clinical team. A few days later I asked how it had gone — Jon cheerily said ‘great! got any more of those phages?’ A few months later, we found out the patient’s infection had been eradicated; for the first time in 3 years, he was off antibiotics. We were thrilled and set out to find phages for the other patients on our growing waiting list.
14 patients treated, and counting
A year has passed since we treated that first patient, and we’ve since treated 13 more using the STAMP protocol here at Westmead Health Precinct. We’ve got a functioning system where Stephanie Lynch in our lab does diagnostics, then hands to me for phage production and purification. I give purified phage back to her for quality control (including sending for sequencing and analysis by our bioinformatician, Nouri Ben Zakour) before diluting to the therapeutic dose and aliquoting it into single-dose vials. We submit our standard set of reports to the local health regulator, send the phage to the patient’s bedside, and the patient gets treated.
At this stage, therapeutic monitoring begins. This includes quantifying phage and bacteria in blood samples, which tells us whether the phages are getting to the infection site and replicating on their target. We’ve also collected additional samples for clinical research (watch this space).
Building a data system to learn from each patient
Collecting data on what happens during phage therapy is hard when every patient gets a different set of phages. There’s lab data and genomics data for each strain and each phage, and there’s clinical data from the patient. Excitingly, Jan has recently completed the build of our clinical trial data capture system, and Stephanie and I are now working with him to do the same for lab data. Jan’s next step will be to integrate the two so we can draw insights about which phages are working, what makes a patient more suitable for phage therapy, and beyond.
Collaborating with regulators
Since the beginning, the Therapeutic Goods Administration (TGA, Australia’s health regulator) has been actively engaged in conversations with the Phage Australia team about how to regulate phage therapy. There’s an understanding that similar to faecal transplants, phage therapy probably needs its own framework. In most countries, there is no formalised regulatory framework for phage products, which tends to make investment into phage therapeutics seem risky. This should change soon, especially for countries with national health payers; health economics assessments are beginning to show cost-effectiveness of introducing phage therapy into a clinical service based on reduced healthcare expenses from preventing surgeries, prosthetic replacements and hospital stays.
Through STAMP, we continue to collect data to show that phage therapy is safe and feasible, keeping regulators in the loop throughout. The Phage Australia team is also working with local government and health districts on a phage therapy reimbursement and procurement model.
Progress and shifting sentiments
Over the last six years, I’ve noticed shifts in phage therapy perceptions and bottlenecks. For researchers, I’ve seen a shift from ‘I doubt I can help’ to ‘what species do you have, and when do you need phages?’ For physicians, it’s gone from ‘would phages even be available?’ to ‘might phages help this patient?’. In fact, we did a survey of Australian prescriber attitudes to phages, and most had patients they would treat right now if they had phages. This survey was also adapted by our collaborator Shinwon Lee for Korean prescribers, showing similar attitudes.
Phage Directory has now sourced phages for more than 50 patients upon clinician request. We’ve built up a community of 1300+ phage researchers from 80+ countries, which collectively have 10,000+ phages against almost 200 species. Our tight-knit community helps each other when needed, and our weekly newsletter/blog, Capsid & Tail, keep everyone connected. Our researchers know what it takes to support phage therapy cases, and know where to come if they need help or phages for any reason.
Phage Australia is now an Australia-wide network with 75+ investigators, with a functioning system for treating 1-2 patients per month, and continues to drive talks with regulators about devising a framework for regulating phage therapy. Recently the team established a successful global clinical phage therapy grand rounds program, and welcomed its first industry-led phage therapy study into the network (DiSArm study). And this July, the New South Wales state government announced $3.5 M to fund operations for another two years.
Phage therapy regulation being discussed in multiple countries
Word about the STAMP protocol and Phage Australia’s model has spread, and groups from Canada, US, UK, Nepal, South Korea, Israel and Singapore are interested in implementing it in their own jurisdictions. They’re especially interested in the clinical-scientific network, regulatory strategy, and phage diagnostic and production infrastructure. One of these groups, led by Greg German at the University of Toronto, has just raised $5M to start a phage therapy centre and clinical trial.
Another interesting trend is that governments are starting to hold formal phage therapy discussions. The first example was a parliamentary hearing in Belgium in 2018, which led to a national framework allowing phages to be produced and quality-certified for patient use. This year, the UK Parliament held a hearing, which I recently learned was a result of the successful ‘My Science Inquiry’ pitch by Applied Microbiology International (on behalf of the phage community, thank you!). Most recently, the European Medicines Agency solicited opinions to guide the design of a regulatory framework for phage products in the veterinary field, which was just released.
Many phage groups around the world, including Phage Australia, were asked to provide evidence for these hearings. It’s been great to see phage researchers and clinicians taking action on this level, and to see that governments are recognizing the need to discuss how to regulate, support and incentivize phage therapy.

It may not fit the existing model, but it still might work
I’m so proud of being part of both Phage Directory and Phage Australia, and look forward to continuing to help push them both forward. I’m also excited to be helping other phage centres get started, from finding out what it takes, to finding the right partners, to setting up diagnostics, production and QC systems, to setting up the lab-clinic interface.
Phage therapy represents hope for many patients combating drug resistant infections. It’s been prohibitively expensive to prove that it works, and almost no one has been confident in its ability to make money. But personalised medicine is becoming more widely accepted. When I talk to people now, they don’t scoff at phage therapy anymore. I mention personalised medicine and complex biologics, and they nod knowingly. Many gave up on phage therapy when it didn’t fit pharma’s one-size-fits-all model, but it’s now becoming clear that phages aren’t the only therapeutics that need a new one.
Our journey from Phage Directory to Phage Australia has taught me a lot about what it takes to make a difference, even in a highly regulated, understandably conservative industry like healthcare. Phage therapy may not be a blockbuster drug, but its viability is gradually being demonstrated, in large part due to the academic labs doing the work to test and produce phages, and doing the clinical trials the field needs. Patients are lining up, their physicians are increasingly on board, and governments are beginning to pay attention.
Resources
- If you’re looking to build a phage therapy center or get involved in phage therapy, email me at jessica@phage.directory. I’m happy to have a conversation, answer your questions, and point you in the direction of the resources you need.
- Phage Directory’s website.
- Phage Australia website.
- Subscribe to Phage Directory’s weekly newsletter and blog, Capsid & Tail to keep up with the phage field.
- Check out my blogs and talks about Phage Australia’s phage therapy system here, here and here.
- More details about the Phage Directory—Phage Australia partnership.
- Khatami et al. BMJ Open paper on the STAMP protocol.
- Khatami et al. EMBO Molecular Medicine paper documenting Dhanvi’s case.
- Petrovic Fabijan et al. Staphylococcus aureus phage therapy paper in Nature Microbiology, which was the predecessor of the STAMP trial.
- New paper on the Belgian ‘Magistral Phage’ regulatory framework.
Thanks very much to Ruby Lin for providing comments on this article!







No comments yet