An experimental treatment made from a plant virus is effective at protecting against a broad range of metastatic cancers in mice, shows a new study from the University of California San Diego.
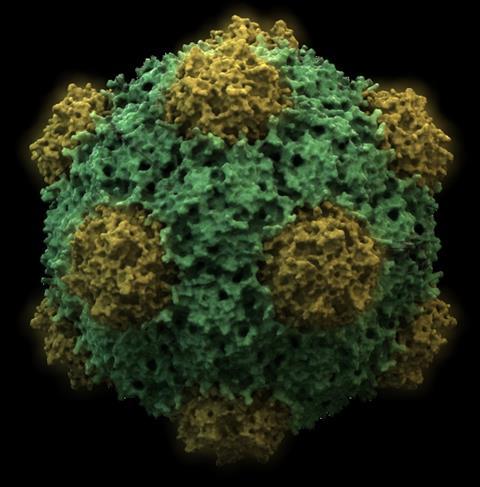
The treatment, composed of nanoparticles fashioned from the cowpea mosaic virus—a virus that infects black-eyed pea plants—showed remarkable success in improving survival rates and suppressing the growth of metastatic tumors across various cancer models, including colon, ovarian, melanoma and breast cancer. Similar outcomes were also observed when the treatment was administered to mice whose tumors were surgically removed.
The findings were published recently in Advanced Science.
Virus nanoparticles
The new study builds upon previous research by the lab of Nicole Steinmetz, a professor of nanoengineering, director of the Center for Nano-ImmunoEngineering and co-director of the Center for Engineering in Cancer, all at UC San Diego. Steinmetz and colleagues have been using cowpea mosaic virus nanoparticles to trigger the immune system to fight cancer and prevent it from spreading and recurring.
In early studies, the approach involved injecting the plant virus nanoparticles directly into tumors to stimulate an immune response. Even though the virus is non-infectious in mammals, the body’s immune cells still recognize it as foreign, triggering a robust immune reaction against the existing tumor, as well as any future tumors.
Now, Steinmetz and her team show that the plant virus nanoparticles do not need to be injected directly into tumors to be effective. Administering the nanoparticles systemically improved survival rates and inhibited metastasis across various cancer types.
Waking the immune system
“Here, we do not treat established tumors or metastatic disease—we prevent them from forming. We are providing a systemic treatment to wake up the body’s immune system to eliminate the disease before metastases even form and settle,” said Steinmetz.
To make the nanoparticles, the researchers grew black-eyed pea plants in the lab and infected them with cowpea mosaic virus. Millions of copies of the virus were grown and harvested in the form of ball-shaped nanoparticles, which required no further modification before use in experiments. “Nature’s powerful nanoparticles, as produced in black-eyed pea plants,” said Steinmetz.
The researchers tested the efficacy of the treatment in mouse models of colon, ovarian, melanoma and breast cancers. Mice injected with cowpea mosaic virus nanoparticles—and then challenged with metastatic tumors a week later—exhibited improved survival rates and reduced tumor growth compared to untreated mice. Even when challenged with new tumors a month later, treated mice exhibited similar outcomes.
Post-surgery effectiveness
The researchers are particularly excited about the treatment’s effectiveness post-surgery. In another set of experiments, administering the nanoparticles after surgical removal of tumors resulted in improved survival rates and decreased tumor regrowth in mice.
“Even if you perform surgery to remove the tumors, no surgery is perfect and there is outgrowth of metastasis if no additional treatment is provided,” said Steinmetz. “Here, we use our plant virus nanoparticles after surgery to boost the immune system to reject any residual disease and prevent circulating tumor cells from metastatic seeding. We found that it works really, really well!”
The goal is to gear up for clinical trials. As the research progresses, the team will be conducting safety studies and exploring the treatment’s efficacy in pet animals with cancer. Future studies will also focus on understanding the mechanisms underlying the immune-boosting properties of cowpea mosaic virus nanoparticles.
Paper: “Systemic Administration of Cowpea Mosaic Virus Demonstrates Broad Protection Against Metastatic Cancers.” Co-authors include Young Hun Chung, Zhongchao Zhao, Eunkyeong Jung, Anthony O. Omole, Hanyang Wang and Lucas Sutorus, all at UC San Diego.
This work was supported in part by the National Institutes of Health (R01-CA224605, R01-CA274640, R01-CA253615) and the Shaughnessy Family Fund for Nano-ImmunoEngineering at UC San Diego.
Disclosure: Nicole Steinmetz is a co-founder of, has equity in, and has a financial interest in Mosaic ImmunoEnginering Inc. Steinmetz is a co-founder of, and serves as manager of Pokometz Scientific LLC, under which she is a paid consultant to Mosaic ImmunoEngineering Inc., Flagship Labs 95 Inc., and Arana Biosciences Inc. The other authors declare no potential conflict of interest.





No comments yet