A team of researchers from Yong Loo Lin School of Medicine, National University of Singapore (NUS Medicine) and Tsinghua University has unveiled a new messenger ribonucleic acid (mRNA) vaccine technology that could make future vaccines safer, more effective, and less burdensome for patients.
Published in Nature Materials, the new approach uses albumin-recruiting lipid nanoparticles, called Evans Blue-modified lipid nanoparticles (EB-LNP), to deliver mRNA precisely to lymph nodes, which are immune system “command centres”, while bypassing the liver, a common site of toxicity for current vaccines. In laboratory tests, the technique outperformed traditional delivery systems in both cancer treatment and viral infection protection, including against melanoma, HPV-related cancers, H1N1 influenza, and Omicron SARS-CoV-2 variants.
READ MORE: Engineered gut bacteria improves survival outcomes in colorectal cancer tumors
“Traditional lipid nanoparticle-based vaccines can accumulate in the liver after intramuscular injection, raising the risk of liver damage and dampening immune responses,” explained Professor Shawn Chen Xiaoyuan, co-senior author of the paper, from the Department of Diagnostic Radiology, and Nanomedicine Translational Research Programme at NUS Medicine. “We aimed to design a smarter delivery system that avoids this problem and directs the vaccine exactly where it’s needed.”
Trapped in the liver
While mRNA vaccines require efficient delivery to immune tissues, conventional polyethylene glycol-lipid nanoparticles (PEG-LNP) systems often get trapped in the liver. LNPs are made at extremely small scales in order to deliver medication or mRNA to our cells. However, repeated high doses, especially in cancer settings, can cause inflammation, anaphylaxis, and even liver damage.
The solution was to use albumin—a natural transport protein in the body. The team engineered a delivery vehicle that hitchhikes on albumin to guide the vaccine payload through the lymphatic system directly to lymph nodes. There, the vaccine is efficiently absorbed and triggers a targeted and powerful immune response.
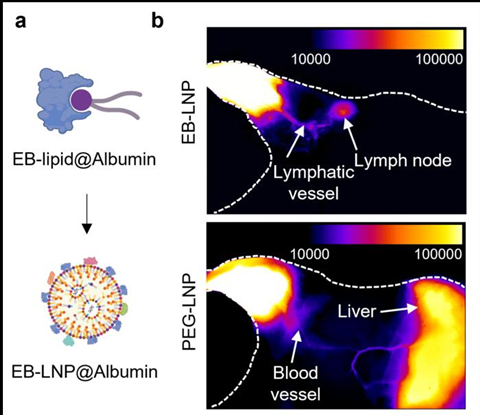
The team synthesised a special Evans Blue-modified lipid (EB-lipid) that binds tightly to albumin. When injected intramuscularly, EB-LNPs recruit albumin to their surface, which naturally guides them to lymph nodes instead of the liver. This design avoids systemic circulation, limiting liver exposure and potential toxicity. Even at lower doses, EB-LNP vaccines produced strong antitumor T-cell responses and high levels of neutralising antibodies. No liver inflammation or toxic responses were observed—even after repeated injections. Unlike traditional PEG-LNPs, EB-LNPs did not trigger strong anti-drug antibodies.
Albumin-hitchhiking strategy
“This albumin-hitchhiking strategy represents a paradigm shift in mRNA vaccine delivery,” said Assistant Professor Guocan Yu, Key Laboratory of Bioorganic Phosphorus Chemistry & Chemical Biology, Department of Chemistry, Tsinghua University. “It has broad implications for cancer, infectious diseases, and potentially autoimmune disorders. For patients, that means fewer injections, reduced side effects, and longer-lasting protection.”
Going forward, the research team is preparing to advance to clinical trials to ensure human safety and efficacy, expand use cases to other diseases, such as autoimmune conditions and lymphatic cancers, and collaborate with the industry to scale manufacturing and accelerate vaccine development.
“Our hope is to transform how mRNA vaccines are designed—making them safer, more effective, and easier to administer,” said Pei Huang, co-lead author of the paper, and Research Fellow at the Department of Diagnostic Radiology, and Nanomedicine TRP at NUS Medicine.






No comments yet