New research being presented at the Letters in Applied Microbiology ECS Research Symposium this May will reveal how scientists are investigating how macrophages can be used to break down amyloid plaques in biofilms.
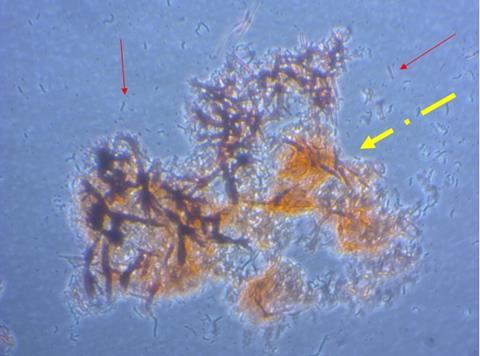
Understanding how macrophages can degrade amyloid will allow us to circumvent pathogen protection by amyloid and help to prevent hospital-acquired infections, says Hy Thai of the University of the West of England, who will present the research.
This research piece is entitled ‘Are synthetic amyloids suitable models for studying Biofilm-Macrophages interaction?’
Biofilm protection
“The presence of large plaques of amyloid in bacterial and fungal biofilms protects them from physical and biological degradation. This allows pathogenic bacteria or fungi to develop in catheters and other areas where they can cause infection,” Hy Thai says.
“Understanding how macrophages can degrade amyloid will allow us to circumvent pathogen protection by amyloid and help to prevent hospital-acquired infections.”
“Our team at UWE is looking at interactions between monocytes or macrophages with amyloid. We are developing an in vitro model with a view to looking at this in vivo, especially regarding amyloid in fungal and bacterial biofilms.”
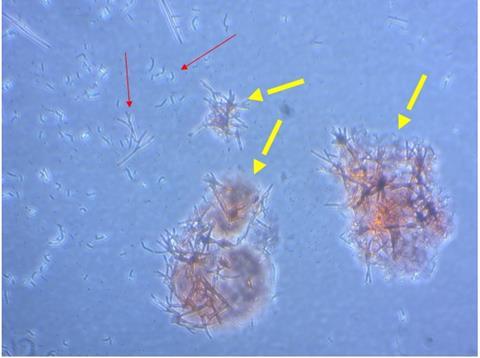
“We have identified conditions for making amyloid in vitro and discovered conditions for differentiating cells of monocyte-macrophage lineage to generate different M1 and M2 macrophage phenotypes and multinucleated cells which might be capable of degrading large amyloid deposits.”
Macrophages and amyloid clearance
“The next stage will be to investigate M2 macrophages mechanisms for breaking down amyloid fibrils through phagocytic activity and investigate if Serum amyloid P (SAP) enhance amyloid resistance to M2 macrophages clearance.”
The human immortalised monocytic cell lines were treated with a phorbol ester and cytokines to differentiate in either a M1 or M2 direction. The characteristics of the treated cells were investigated in assays for phagocytosis of microbeads and synthetic amyloids, including oligomeric and fibrillar structures.
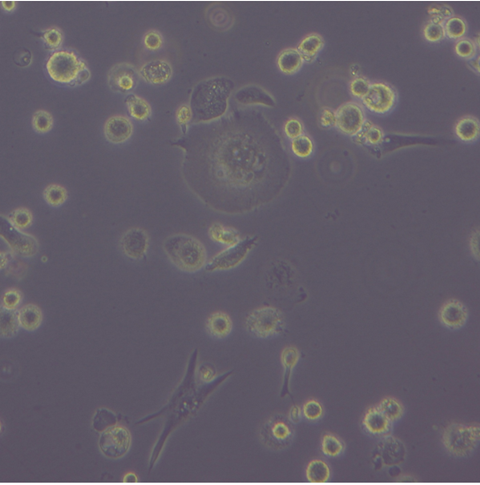
The team found a high cytotoxicity of synthetic amyloid at the concentration of 1µM and 5µM. Furthermore, the interaction between anti-inflammatory M2 macrophages and amyloids resulted in a significant increase in expression CD36 and CD209, and slight production of IL-10 while expression of TNF-a cytokines remained low.
Potential shift
“This might signify the potential shift in M2 macrophages’ function or phenotypes, demonstrating their attempt to engulf and clear the amyloid deposits. This model could give us an idea on how M2 macrophages would interact with fungal amyloids,” Hy Thai said.
“Synthetic amyloid exposure at 1µM and 5µM showed a strong cytotoxicity in macrophages. Furthermore, we found the interaction between anti-inflammatory macrophages and amyloids resulted in a significant increase in expression CD36 and CD209, and production of IL-10 cytokine remained low while TNF-a expression was minimal. These observations suggested a potential shift in function of M2 macrophages, possibly reflecting their attempts to phagocyte and clear up synthetic amyloid deposits. This in vitro model provided us valuable insights into how M2 macrophages might interact with fungal amyloids.”
Surprise finding
The ability of macrophages differentiated into one phenotype to exhibit plasticity and convert or revert to other phenotypes was unexpected, as the M1 and M2 phenotypes have been regarded as terminal states of differentiation. The findings suggest that homeostatic mechanisms can restore the balance between cells of different phenotypes and limit immune responses which are selectively promoted by cells of each phenotype, Hy Thai said.
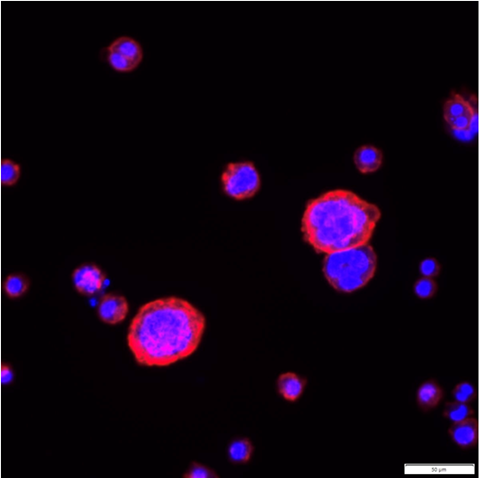
“This work means that we need to look more closely at the way in which macrophages interact with amyloid deposits, especially those which contain serum amyloid P component (SAP). We need to know whether and how the presence of SAP changes the phenotype of macrophages to an unproductive state.”
Paving the way
“Removal of SAP has been posited as a step which enables macrophages to interact productively with human amyloid, such as that found in brain plaques in Alzheimer’s disease, and fungal amyloid (Candida albicans).”
“The first step is to characterise the molecules on macrophages which interact directly with amyloid and SAP and determine pathways by which changes in macrophage phenotype are affected.”
“More works to be done using primary human monocytes to see whether these results can be replicated and investigate the molecular mechanism underlying the interaction between human innate immune cells (e.g., primary macrophages) with Candida albicans-derived fungal amyloid.”
The work was performed at UWE by PhD student, Hy Gia Thai, under the supervision of Dr Trevor Whittall and Dr Robin Thorn with advice in the early stages of the project from Professor Myra Conway, now at the University of Derby.
The Letters in Applied Microbiology Early Career Scientist Research Symposium will be held at University of the West of England (UWE) in Bristol, UK, on 15 May 2024. To find out more, visit the event page.
Topics
- Alzheimer’s disease
- Applied Microbiology International
- Bacteria
- Biofilms
- Candida albicans
- Community
- Early Career Research
- Fungi
- Hy Gia Thai
- Infection Prevention & Control
- macrophages
- Myra Conway
- One Health
- Research News
- Robin Thorn
- Serum amyloid P
- Synthetic Biology
- Trevor Whittall
- UK & Rest of Europe
- University of the West of England






No comments yet