Chronic hepatitis B (CHB) poses a major global health burden, with China particularly affected. Effective antiviral therapy is crucial to prevent disease progression, but responses may vary by Hepatitis B virus (HBV) genotype.
This prospective study aimed to compare genotype-specific responses to 144-week entecavir (ETV) therapy in HBeAg-positive CHB patients, with particular emphasis on histological improvement assessed through paired liver biopsies.
The authors enrolled 49 treatment-naïve CHB patients (HBV DNA ≥ 20,000 IU/mL, alanine transaminase (ALT) > 2× ULN, and Scheuer system G ≥ 2) who received ETV 0.5 mg/day. HBV genotyping was performed using Polymerase Chain Reaction and fragment length analysis. The primary endpoint was histological improvement (i.e., ≥ 2-grade reduction in necroinflammatory activity without fibrosis progression), evaluated via paired biopsies (baseline and week 144) by blinded pathologists. Secondary endpoints included virological response (i.e., serum HBV DNA < 100 IU/mL), HBeAg seroconversion, and ALT normalization.
Results
The cohort included 24 genotype B and 24 genotype C patients (one genotype A patient was excluded from genotype-specific analyses). Genotype B showed significantly higher histological improvement rates (91.3% vs. 63.2%, P = 0.027) and greater inflammation resolution (0 ≤ G < 1: 56.5% vs. 26.3%, P = 0.048).
Virological suppression was excellent in both groups (100% vs. 100%). HBeAg seroconversion trended higher in genotype C (29.2% vs. 50.0%, P = 0.140). All patients achieved ALT normalization by week 48, with no safety concerns.
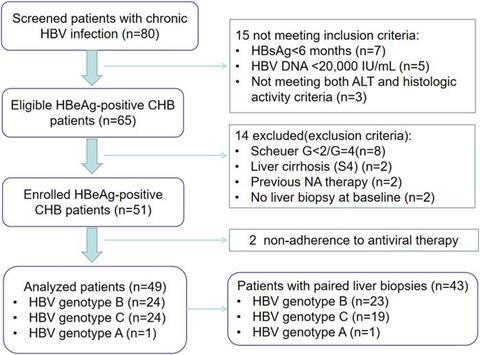
HBV genotype significantly influences liver histological response, with patients with genotype B exhibiting superior improvement in necroinflammation compared with those with genotype C. These findings provide compelling evidence to support the incorporation of HBV genotyping into routine clinical practice, as it can serve as a critical determinant for predicting histological responses and thus should be considered when formulating individualized therapeutic strategies for CHB patients. Standardization of follow-up and systematic endpoint assessment would further strengthen the validity and clinical relevance of the present study.
The study was recently published in the Journal of Clinical and Translational Hepatology.






No comments yet