Pus, strep throat, and even tuberculosis—most infectious diseases are characterized by a cluster of pathogenic bacteria that can be stubborn and resistant to antibiotics. Researchers from Ben-Gurion University of the Negev have found another method to combat these bacteria using naturally sourced molecules found in corals. The research findings were published in the journal BMC Biology last month.

Groups of bacteria in a fixed location recognize each other through communication based on chemical molecules. Based on this communication method, called ‘quorum sensing,’ a research group led by Prof. Ariel Kushmaro, with Dr. Karina Golberg from the Environmental Biotechnology Lab in the Avram and Stella Goldstein-Goren Department of Biotechnology Engineering at Ben-Gurion University of the Negev, turned to nature and asked: Can these communication properties be used to inhibit harmful bacteria?
READ MORE: Derivative of the long pepper battles bacterial biofilms
READ MORE: Natural molecule added to toothpaste may help prevent plaque and cavities
Biofilm is a “glue-like” layer characterized by a high concentration of bacteria that can communicate with each other, thereby creating protection from the environment, including antibiotics and the immune system. To try and “break” this defense, over 120 bacteria living on corals near the Interuniversity Institute for Marine Sciences in Eilat were collected and isolated. After laboratory tests, the researchers successfully found chemical molecules from the bisindole (BISINDOLE) group in the bacteria that alter the properties of harmful bacteria and their ability to form biofilms.
Molecules from coral
“The bacteria we found on corals secrete these molecules, which selectively target the virulence of harmful bacteria,” says Dr. Golberg. “The molecules found are unique in that they weaken the ability of resistant bacteria to cause harm without killing them. The molecules disrupt properties controlled by bacterial communication, thereby helping prevent these bacteria from producing antibiotic-resistant biofilm. In addition, the molecules reduce the toxicity of these bacteria, their virulent properties, and even make antibiotics (such as tobramycin) work better, ultimately killing the bacteria even when they form a biofilm.”
The study examined four pathogenic bacterial strains known for their high antibiotic resistance and their ability to cause severe infections, mainly in hospitals. The research itself focused on two main bacteria—Pseudomonas aeruginosa and Acinetobacter baumannii—which received special attention due to their high lethality and direct link to chronic infections in the lungs and bloodstream. The combination of bisindole molecules with the bacteria significantly reduced the virulence of the bacteria, and in combination with antibiotics (tobramycin), it was found that one of the molecules increased antibiotic penetration into the biofilm, allowing for more effective killing of the bacteria.
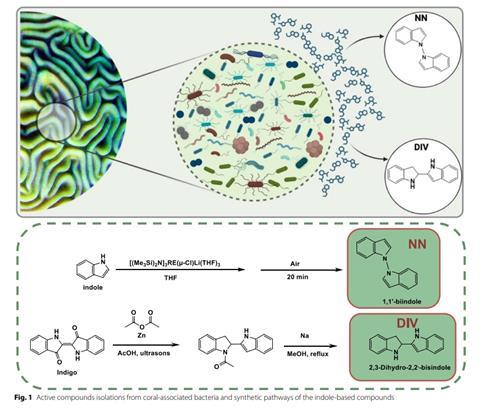
“The central innovation of the study is the development of a new treatment strategy for pathogenic bacteria that allows for the modification of the bacteria’s virulent properties without killing them. Until now, treatments using antibiotics cause the death of bacteria and/or an increase in antibiotic resistance and can also harm beneficial bacteria that are part of the human microbiome,” explained Prof. Kushmaro.
Disrupting chemical communication
The research results present an innovative approach where the combination of natural substances that disrupt chemical communication in bacteria can help us tackle antibiotic-resistant bacteria. The research advances us one step further towards the development of new and effective drugs that can act without harming essential bacteria.
The research group included: Karina Golberg, Bat-El Kagan, Marilou Shagan, Netta Shemesh, Esti Kramarsky-Winter, Anat Ben-Zvi, Yaffa Mizrahi Nebenzahl z”l, Robert S. Marks, Ariel Kushmaro from Ben-Gurion University of the Negev, and Kamal Elouarzaki from the School of Materials Science and Engineering at Nanyang Technological University, Singapore.
This work was supported by the National Institute of Biotechnology in the Negev (NIBN), with financial support under grant number 8528620 “Bioactive compounds.”






No comments yet