Penile squamous cell carcinoma (PSCC) is a rare but aggressive malignancy that severely affects men’s health worldwide. Half of all PSCC cases are linked to human papillomavirus (HPV) infection, yet the biological basis for the better prognosis of HPV-positive tumors remains unclear.
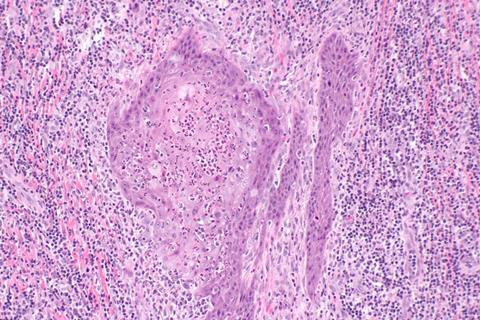
Using single-cell RNA sequencing on tumor samples from 11 patients, researchers profiled nearly 53,000 individual cells to reconstruct the tumor microenvironment. They discovered that HPV-positive tumors contained fewer proliferative macrophages and less exhausted CD8+ T cells, along with stronger chemokine signaling. These findings reveal distinct immune remodeling associated with HPV infection and provide new insights into how viral status influences cancer immunity and patient outcomes.
Although penile cancer represents less than 1% of male malignancies, it carries substantial physical and psychological burden, with a five-year survival rate near 50%. Around half of Penile squamous cell carcinoma (PSCC) cases are associated with persistent HPV infection, which triggers oncogenic transformation via viral oncoproteins E6 and E7.
Interestingly, HPV-positive PSCC patients tend to have better survival and treatment responses compared to HPV-negative cases. However, the mechanisms behind this clinical advantage remain poorly understood. Due to limited patient samples and tumor heterogeneity, previous genomic studies failed to fully capture the immune microenvironment. Given these challenges, it is necessary to explore the cellular ecosystem of HPV-associated PSCC using single-cell approaches.
Tumor microenvironment
A research team from the University of Science and Technology of China has mapped the tumor microenvironment of HPV-associated penile cancer at single-cell resolution. The study, published (DOI: 10.1093/pcmedi/pbaf013) in Precision Clinical Medicine in September 2025, analyzed 52,980 single cells from 11 treatment-naïve PSCC patients.
By comparing HPV-positive (HPV+) and HPV-negative (HPV−) tumors, the researchers revealed profound immune differences that may explain variations in prognosis and therapeutic response. The single-cell atlas provides a high-resolution reference for understanding how HPV infection shapes tumor immunity and microenvironmental dynamics.
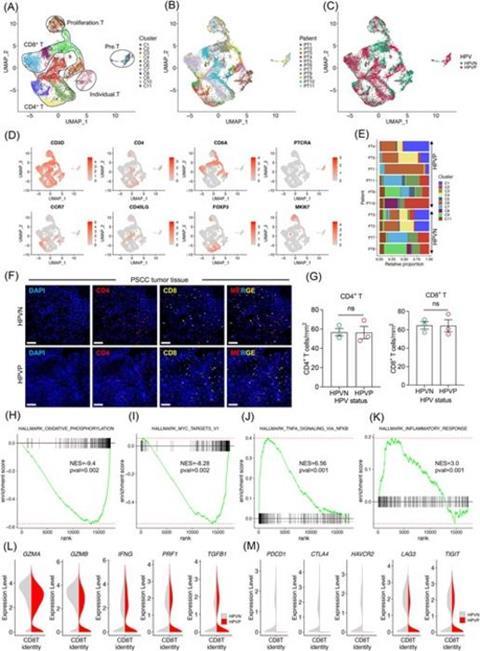
Using high-throughput single-cell RNA sequencing, the team profiled immune, stromal, and epithelial cells from PSCC tissues to dissect their heterogeneity. Unsupervised clustering identified 49 distinct cellular subpopulations grouped into seven major cell types, including T cells, B cells, macrophages, NK cells, endothelial cells, and cancer-associated fibroblasts (CAFs).
Notably, HPV+-PSCC tumors showed an increased abundance of mast cells but a significant reduction in proliferative macrophages compared to HPV− cases.
Gene ontology analysis
Gene ontology analysis revealed that inflammatory CAFs in HPV+-PSCC expressed higher levels of chemokines such as CXCL13, CXCL3, and CXCL12, suggesting enhanced immune recruitment. In contrast, antigen-presenting cells in HPV− tumors upregulated CXCL5, CXCL1, and CCL13, promoting leukocyte migration and a more immunosuppressive niche.
Transcriptomic profiling further revealed that CD8+ T cells in HPV+-PSCC expressed markedly lower levels of immune checkpoint molecules—including PDCD1 (PD-1), CTLA4, HAVCR2 (TIM-3), LAG3, and TIGIT—indicating reduced exhaustion and potentially greater antitumor activity. Ligand–receptor analysis highlighted that inhibitory signaling via TIGIT and its ligands (PVR, NECTIN2/3/4) was enriched in HPV− tumors, reinforcing their suppressed immune environment.
Remodeling the tumor ecosystem
“Our findings provide a comprehensive single-cell atlas of PSCC and uncover how HPV infection remodels the tumor ecosystem,” said the study’s investigators from the University of Science and Technology of China. “By identifying cell-type-specific immune signatures and signaling interactions, we can better understand why HPV-positive tumors tend to respond more favorably to therapy. These results also emphasize the importance of examining viral factors when designing personalized immunotherapy strategies for rare cancers like PSCC.”
This single-cell landscape offers a crucial resource for future research on viral-induced cancers and tumor immunology. The discovery that HPV-positive tumors harbor fewer exhausted CD8+ T cells and weaker inhibitory TIGIT–PVR signaling suggests potential biomarkers for prognosis and targets for immunotherapy.
Therapeutic strategies aimed at restoring immune activation in HPV− tumors—such as TIGIT blockade or chemokine modulation—could enhance treatment efficacy. Beyond penile cancer, this study - published in Precision Clinical Medicine - also provides a conceptual framework for understanding how viral infections shape tumor immune microenvironments across different cancer types.





No comments yet