As the FDA issues warning letters about probiotic products sold for use in hospitalized preterm infants, families of babies threatened by necrotizing enterocolitis (NEC) now face a deadly Catch-22 situation.
Food and Drug Administration (FDA) regulations describe a distinction between a food, a dietary supplement, and a drug or vaccine; this distinction is in part determined by intent.
If a food or a dietary supplement is intended to diagnose, treat, mitigate, cure, or prevent a disease or condition it must move from the category of a food or a dietary supplement to the category of a drug or vaccine with a higher degree of oversight to ensure safety and efficacy.

The challenge comes when evidence mounts that a particular food or dietary supplement actually treats or prevents a disease, but the manufacturer prefers to keep this product in the category of a food or dietary supplement to avoid the challenges and costs of the higher level of oversight.
The result is that one mission of the FDA (protecting public health by assuring the safety, efficacy, and security of products) precludes the other mission of the FDA (providing accurate, science-based health information to the public). In addition, manufacturers share limited data with clinicians and the public to avoid being penalized, and clinicians are stifled from open and transparent communication with patients and families.
Necrotizing enterocolitis
The first cohort study demonstrating benefit of probiotics for the prevention of necrotizing enterocolitis (NEC) was published in 1999. Since then 60 randomized trials of probiotics including 11,156 preterm infants have been published demonstrating a significant decrease in the risk of NEC (risk ratio (RR) 0.54 (95% CI 0.46, 0.65) number needed to treat for benefit 33) and all-cause mortality (RR 0.77, 95% CI 0.66, 0.90, NNTB 50).
In addition, meta-analysis of 30 published observational cohort studies of probiotics including 77,018 preterm infants showed similar improvements in reduction of NEC (odds ratio 0.60 95% CI 0.50, 0.73) and death (OR 0.77 95% CI 0.68, 0.88). Based on these studies, the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition, the American Gastroenterological Association and the World Health Organization issued conditional recommendations for provision of probiotics to preterm infants for the prevention of NEC, while the American Academy of Pediatrics recommended that “Centers making the decision to administer probiotics to select preterm infants should discuss the potential risks and benefits of this therapy with parents ….”
Warning letter
In September 2023, the FDA issued a letter to healthcare providers warning that “preterm infants are at risk of invasive, potentially fatal disease caused by the bacteria or fungi contained in probiotics” after the death of an extremely low birth weight preterm infant who developed sepsis caused by a probiotic bacterium.
The letter reminded healthcare providers of the need to submit an investigational new drug application (IND) to the FDA if they administer probiotics to “treat, mitigate, cure or prevent a disease or condition….” Subsequently, FDA forced two widely used probiotic products off the market, not because of evidence of contamination or non-adherence to Good Manufacturing Practices, but because marketing materials noted evidence from clinical studies that probiotics decrease the risk of NEC, death, and sepsis. The mention of these clinical studies moved the products from a dietary supplement to an unapproved new drug and an unlicensed biological product.
Catch-22
A ”Catch-22” refers to a situation in which the rules deny a solution. For 20 years, the study of probiotics for preterm infants in the United States has been limited due to the following Catch-22:
1) since compelling evidence shows a reduction in NEC and death, probiotics must be viewed as drugs which requires premarket review and demonstration of safety and effectiveness plus ongoing manufacturing and testing standards for drugs
2) probiotic manufacturers prefer to remain in the category of dietary supplements (rather than drugs) given the costs involved and their primary market (not preterm infants)
3) NIH is not able to fund clinical trials of probiotics without IND oversight by the FDA
4) clinicians and researchers are unable to submit an IND application without data from manufacturers regarding purity, viability, and manufacturing standards that are the equivalent of a drug or vaccine.
Probiotic use
This Catch-22 is unique to the United States. Probiotic administration to preterm infants is common in much of the world but probiotic use in U.S. NICUs dropped dramatically after the FDA’s warnings, despite strong evidence that probiotics decrease the risk of death in extremely preterm infants.
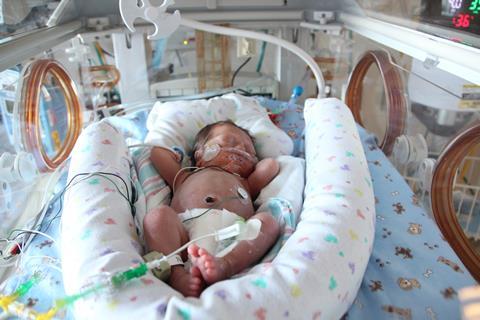
The FDA has not clarified what level of increase in the mortality rate in very preterm infants they deem acceptable with the cessation of probiotic administration in the 30% of U.S. neonatal intensive care units (NICUs) previously administering probiotics.
Paradigm shift
Catch-22 situations are only resolved with a paradigm shift. The following potential solutions have been proposed:
1) a probiotic manufacturer could produce a probiotic to be marketed as a drug and manufactured to the same standards as a vaccine (there is one such product that has advanced to a phase 3 multi-center clinical trial which should be completed in 2024 at a total cost thus far of $100M)
2) the FDA could take an approach similar to that of other countries and create a category for dietary supplements that contain live organisms that differs from that of drugs or vaccines (e.g. the Canadian approach of categorizing probiotics as natural health products if a health claim is made allows regulation and use across Canadian NICUs), This would encourage manufacturers of probiotics following current Good Manufacturing Practices to share their data with clinicians and researchers without being penalized. Investigators could then submit an IND application allowing FDA oversight to ensure safety and efficacy. Clinicians could share data with parents without fear of reprisal (current FDA regulations allow a clinician to tell parents that mom’s milk and/or probiotics “improve gut health” but not that both have been shown to decrease the risk of NEC).

Most treatments routinely provided to extremely preterm infants are not FDA-approved for specific indications, and clinicians, who have different goals than regulators, rely on available evidence to guide treatment decisions.
Every day we wait for a paradigm shift, more babies develop and die from NEC. NICU families are eager for more information about how they can protect and nurture their newborn’s health, and yet the ability to provide accurate information and the most protective clinical care is being hindered by the FDA. Clinicians and families affected by NEC are eager to work with the FDA and industry to resolve this deadly Catch-22.
Mark A. Underwood MD is Emeritus Professor of Pediatrics at UC Davis School of Medicine.
Jennifer Canvasser founded the NEC Society in January 2014 after the loss of her 11-month old son Micah. Micah developed NEC when he was six weeks old, which resulted in a host of life-threating complications. Micah passed away from these complications in December of 2012.
Jae Kim MD is a neonatologist and pediatric gastroenterologist and division chief at Cincinnati Children’s.






No comments yet