Researchers at Mass General Brigham have modified a herpes simplex virus (HSV-1) that stimulates the immune system to attack glioblastoma cells. A single dose of the modified virus increased T-cell, natural killer cell, and myeloid cell responses in the tumor microenvironment and increased the overall survival in preclinical models. Results are published in Nature Cancer.
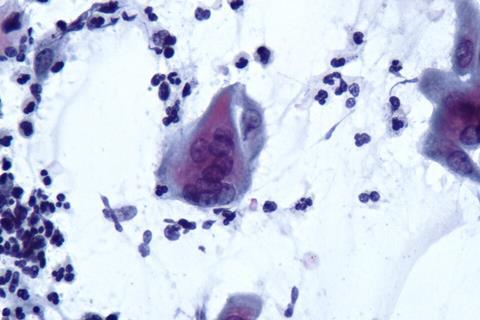
Glioblastoma is among the most aggressive and treatment-resistant brain tumors. Previous attempts to stimulate the immune system to attack tumor cells in the brain have shown limited benefit, in part because glioblastoma cells release multiple molecules that dampen immune responses.
READ MORE: New herpes virus–based vaccine could cure cancer in the future without side effects
READ MORE: Genetic ‘Trojan horse’ selectively kills cancer cells linked to Kaposi’s sarcoma
To overcome the barriers, researchers modified an HSV-1 virus to recognize markers found only on glioblastoma cells. They engineered the virus to express five different immunomodulatory molecules to help reprogram the tumor environment, including IL-12, anti-PD1, a bispecific T cell engager, 15-hydroxyprostaglandin dehydrogenase and anti-TREM2.
Researchers also added safety mutations, or “off-switches,” that prevent the virus from spreading to neurons or healthy central nervous system cells. So that the reach of the virus could be visualized on PET scan, the team inserted a gene that expresses a protein capable of trapping a PET-tracer molecule.
Tumor fighting T cells
Mice treated with the virus showed increased infiltration of tumor-fighting T cells, as well as reduced T-cell exhaustion markers. Mice injected with the virus also lived longer than glioblastoma-harboring mice not injected with the virus.
“We engineered a safe and traceable oncolytic virus with strong cytotoxic and immunostimulatory activities for glioblastoma immunotherapy,” said Francisco J. Quintana, PhD, of the Mass General Brigham Department of Neurology and senior author on the federally funded study. “This platform offers a multipronged approach—precise tumor targeting, local delivery of immunotherapeutic payloads, and a built-in safety system to protect normal brain cells.”
Future research will focus on evaluating the safety and efficacy of the oncolytic virus in human trials as well as adapting the viral platform to remodel the tumor microenvironment in other cancers.
Authorship: In addition to Quintana, Mass General Brigham authors include Federico Giovannoni, Camilo Faust Akl, Brian M. Andersen, Zhaorong Li, Hong-Gyun Lee, María Florencia Torti, Joseph M. Rone, and Pere Duart-Abadia. Additional authors include Craig A. Strathdee, Martina Molgora, Linxing Kong, Michael Floyd, Jian Teng, Yulia Gyulakian, Peter Grezsik, Terry Farkaly, Agnieszka Denslow, Sonia Feau, Irene Rodriguez-Sanchez, Judith Jacques, Marco Colonna, Edward M. Kennedy, Tooba Cheema, Lorena Lerner, and Christophe Quéva.
Disclosures: Strathdee, Kong, Akl, Teng, Gyulakian, Grezsik, Farkaly, Denslow, Feau, Jacques, Rodriguez-Sanchez, Kennedy, Cheema, Lerner, and Quéva were employees of Oncorus during the performance of some of these studies.





No comments yet