Researchers at the State University of Campinas (UNICAMP) in Brazil, in collaboration with colleagues at Harvard University in the United States, have conducted an innovative study of the DNA and population dynamics of Saccharomyces cerevisiae, the yeast used to produce fuel ethanol from sugarcane by plants throughout Brazil, with the aim of finding routes to more stable, consistent and predictable fermentation performance.
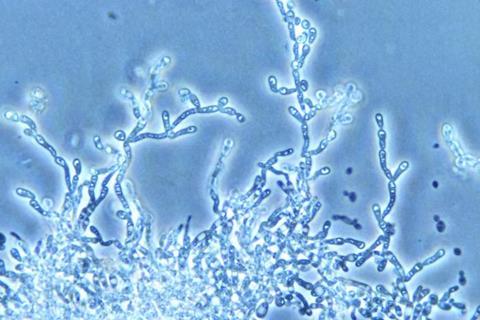
The study was based on whole-genome sequencing and metagenomics, the analysis of a collection of genomes from a community of organisms. The results were reported in an article published in the journal G3: Genes, Genomes, Genetics.
Although S. cerevisiae (brewer’s yeast) is predominant, invasion by foreign strains remains common, as fermentation conditions are affected by factors such as sugarcane variety, process design, operating conditions and weather, not to mention evolutionary change over the course of a fermentation season, making each of the 400 industrial sugar and ethanol plants in Brazil a unique ecosystem.
Wild strains may be brought into the industrial environment by insects or birds from very different environments. Bacterial contamination also affects fermentation in ways that are not fully understood.
Tracking population dynamics
In the study, which was funded by FAPESP, scientists at UNICAMP’s School of Food Engineering (FEA) and Harvard’s Department of Organismic and Evolutionary Biology tracked yeast population dynamics in two biorefineries during two production seasons (April-November 2018 and April-November 2019).
In addition to using their novel statistical framework on a combination of metagenomic and clonal sequencing data for some 150 clones from these biorefineries, they mined data for more than 1,000 strains of the yeast from a European study published in 2018, in order to construct a phylogenetic tree.
“DNA sequencing is like fingerprinting. We use it to identify the microorganisms in ethanol fermentation,” said Andreas Gombert, a professor at FEA-UNICAMP and first author of the article. “We found that despite the presence of invasive strains and the non-aseptic nature of fermentation, all bioethanol lineages were clustered on the same branch of the tree. They were all related and belonged to the ethanol fermentation environment. This is probably why the main indicators of the process remained very stable in both production seasons.”
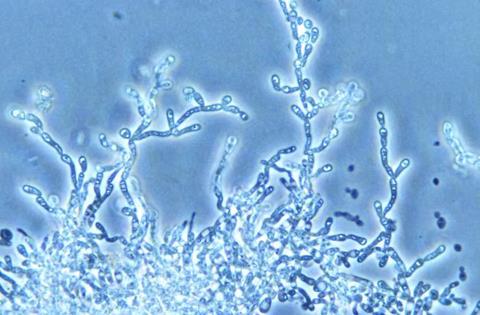
No reproducible pattern
All four scenarios analyzed were found to be different, with no reproducible pattern. In one, all lineages present during the entire production period derived from one of the starter strains, while in another, invading lineages took over the population and displaced the starter strain. The differences between the two consecutive production periods for the same biorefinery were less pronounced than the differences between the two industrial units.
Having obtained these results, the researchers want to understand yeast population dynamics in more detail. For example, which adaptations make each lineage fitter to survive in the fermentation environment in each biorefinery and why do others disappear? What is the effect of external agents that contaminate the process, such as bacteria?
“To find out, we will invest in analysis of not only yeast DNA and but also the DNA of the bacteria that contaminate the process, and then correlate the dynamics of both. This is what we call ethanol fermentation ecology,” Gombert said.







No comments yet