
In a study published in Nature Communications, researchers from the Yong Loo Lin School of Medicine, National University of Singapore (NUS Medicine), together with international collaborators, examined whether methylene blue could mitigate brain injury during severe malaria, and whether a practical set of blood biomarkers could help clinicians identify cerebral malaria early and track how patients respond to treatment.
Assistant Professor Benoit Malleret, Department of Microbiology and Immunology, and Immunology Translational Research Programme (TRP), NUS Medicine, said, “Cerebral malaria develops quickly and leads to severe consequences, but there remains a lack in practical diagnostic tools or targeted therapies. Our findings show that methylene blue can reverse many of the infection-induced molecular changes in the brain, which is encouraging for a compound that is already inexpensive and widely accessible.”
Methylene blue
In the study, the researchers applied methylene blue intravenously in laboratory models with Plasmodium coatneyi after severe symptoms appeared. P. coatneyi closely resembles P. falciparum as it has extremely similar symptoms, and is widely used as a stand-in in laboratory and preclinical studies.
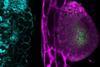
The team then analysed which genes were switched on or off during infection. They used these data to identify patterns in the blood that reliably signalled when cerebral malaria was present. They found that methylene blue was able to reset many of the abnormal genetic changes in the brainstem, the part of the brain most affected during cerebral malaria.
READ MORE: Human antibodies could prevent the malaria parasite from causing life-threatening infections
It also reduced clear signs of brain injury, such as pigment deposits from infected red blood cells, small bleeds, and swelling. Many gene activity changes caused by the infection returned close to normal, and the tissue samples showed improvements that matched the molecular results.
Blood signature
By comparing results across datasets, the researchers identified a nine-gene blood signature: MAG, IL1RN, LCN2, S100A8, S100A9, CD177, CHIT1, MMP9 and NFE2. This set of genes consistently separated cerebral malaria from milder malaria and from healthy individuals.
As the pattern appeared stable across both adults and children, it raises the possibility of developing a single, standardised blood test to help doctors diagnose cerebral malaria, assess its severity, and track patient recovery. Several of these genes are linked to neutrophils, a type of white blood cell, suggesting that neutrophil-driven inflammation contributes to brain swelling and damage during the disease.
Asst Prof Malleret added: “The biomarker signature we identified was remarkably consistent. This suggests that a simple blood test could be developed to differentiate cerebral malaria from other severe conditions, enabling earlier intervention and clearer treatment decisions.”
Immune processes
Another important finding was the improved understanding of immune processes involved in brain injury. Neutrophils, which were not previously considered central to cerebral malaria, appeared repeatedly across the biomarker and immune-cell analyses. This provides new clues about how inflammation damages the blood–brain barrier and how neurological symptoms arise.
While methylene blue showed encouraging effects, the researchers noted that timing may be key, as earlier treatment appeared more beneficial. Clinical trials will be needed to determine the best dosing, timing, and safety when used together with current antimalarial drugs. The nine biomarkers will also need to be tested in larger and more diverse patient groups, and translated into a practical, field-ready test.
The team ultimately hopes to develop a rapid, reliable blood test for cerebral malaria and to evaluate methylene blue as an affordable supportive treatment. By clarifying how the brain becomes inflamed during malaria, the study lays groundwork for better diagnostics, treatments, and patient monitoring in regions where the disease remains a major health concern.





No comments yet