Antibiotic resistance is one of the most pressing challenges to global public health as harmful microbes evolve to evade these medications. Now, researchers at University of California San Diego and their colleagues have developed a new method to combat antibiotic-resistant bacteria using bacteriophages, or phages, for short — viruses that infect and kill bacteria — as an alternative to traditional antibiotics.
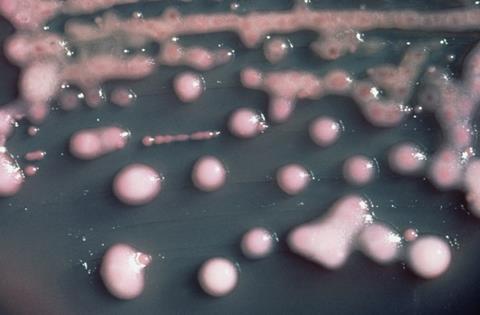
The researchers targeted Klebsiella pneumoniae, a species of bacteria notorious for its ability to resist multiple antibiotics. The dangerous pathogen can cause severe infections in hospital settings, including pneumonia and sepsis. While phages have been used as a treatment for bacterial infections for over a century, they are extremely specific about which strains of a bacterial species they will attack. This has limited their effectiveness against the most antibiotic-resistant strains.
To overcome this problem, the research team “trained” the phages by allowing them to evolve together with the bacteria in a controlled laboratory setting for 30 days.
Experimental evolution
This technique, called “experimental evolution”, permitted the phages to adapt to bacterial defenses. This resulted in significant improvements to their ability to kill a wide variety of bacterial strains, including multidrug-resistant and extensively drug-resistant K. pneumoniae — strains that pose a significant challenge to modern medicine.
What’s more, the evolved phages also demonstrated an enhanced ability to suppress bacterial growth over extended periods of time.
Genetic analysis revealed that the evolved phages acquired mutations to specific genes responsible for recognizing and binding to bacterial cells to initiate the infection process. These changes likely contributed to their improved effectiveness.
The research, led by senior author David T. Pride, M.D., Ph.D., professor of pathology at UC San Diego School of Medicine, highlights the potential of phage therapy as a powerful tool to address the global antibiotic resistance crisis. The team believes their method could be adapted to target other resistant pathogens, offering an avenue for developing treatments against a wide range of life-threatening infections.
The study was published in Nature Communications on November 19, 2025.




No comments yet