The R21/Matrix-M malaria vaccine generates nearly identical antibodies as those following a natural infection, helping explain why the vaccine confers such strong protection against the earliest life stage of malaria parasites, according to research published in the Journal of Experimental Medicine.
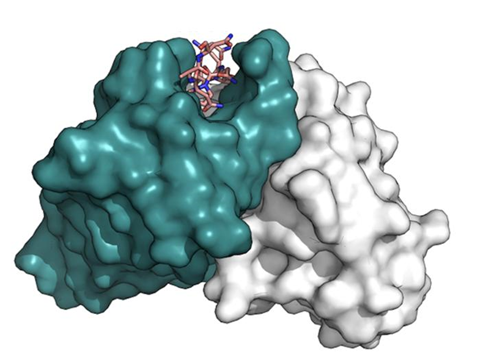
“This is exactly what you want to see – that a vaccine elicits the same immune response as a natural infection, but without the malaria parasites causing disease, which still claims more than 600,000 lives a year,” said Gregory Ippolito, Ph.D., associate professor at Texas Biomedical Research Institute, who led the analysis.
READ MORE: Chemical trick could turn losing malaria drug into a winner
READ MORE: Revealed: New vaccine target to block malaria transmission
The R21/Matrix-M malaria vaccine is one of two malaria vaccines recommended by the World Health Organization for prevention of malaria in children. The R21/Matrix-M vaccine was developed by the University of Oxford’s Jenner Institute and the Serum Institute of India.
To generate protective antibodies, the vaccine mimics parts of the proteins that encase the parasites when they are transmitted from mosquitoes when they bite. It is important to block the parasites at this early sporozoite stage before they spread to the blood stream, which is when symptoms begin. The vaccine also includes Novavax’s Matrix-M adjuvant, which helps boost the immune response.
Dr. Ippolito has been collaborating with Professor Katie Ewer, Ph.D., co-corresponding paper author, and the team developing the R21 vaccine for many years. Studies evaluating the antibody response were completed at University of Texas at Austin during the first human clinical trials.
Probing the antibodies
To identify and quantify antibodies found throughout the body, the researchers used specialized next-generation sequencing and mass spectrometry protocols that Dr. Ippolito codeveloped, called BCR-seq and Ig-seq. These protocols enabled the team to precisely identify the antibodies present in adult volunteers after vaccination and after being bitten by malaria-carrying mosquitoes in a “controlled human malaria infection.” (The strain of malaria was known to be treatable with the medication chloroquine if needed.) The researchers also compared the results to other preexisting data from natural malaria infections.
The results were striking in how the antibodies generated by the R21/Matrix-M vaccine were indistinguishable from those observed following natural infection. The variety of antibodies also did not change following infection in the vaccinated individuals, which indicates they are effective, long-lasting and did not require further adaptation.
“This vaccine approach, including the adjuvant from Novavax, are important to consider as malaria vaccines targeting other life stages of the parasites are developed,” Dr. Ippolito said.
Learn more about Dr. Ippolito’s research and sequencing tools, BCR-seq and Ig-seq, which can be applied for understanding a wide variety of chronic and infectious diseases and exploring new treatments and vaccines.
Topics
- BCR-seq
- Gregory Ippolito
- Ig-Seq
- Immunology
- Infection Prevention & Control
- Infectious Disease
- Katie Ewer
- malaria
- Matrix-M
- Novavax
- One Health
- Parasites
- R21/Matrix-M malaria vaccine
- Research News
- Serum Institute of India
- Texas Biomedical Research Institute
- University of Oxford
- University of Texas at Austin
- USA & Canada
- Vaccinology
- Veterinary Medicine & Zoonoses






No comments yet