Isoflavonoids such as formononetin, calycosin, and calycosin-7-glucoside are major active components of Astragalus membranaceus (Huangqi), a medicinal plant widely used in traditional and modern health products. These molecules are linked to antioxidant, anti-inflammatory, and cardioprotective benefits, driving growing demand worldwide.

However, plant cultivation is slow, sensitive to environmental conditions, and yields are limited even when advanced techniques such as hairy-root cultures or UV induction are applied. In contrast, engineered microbes have already achieved high-level production of related flavonoids within days rather than months.
Despite this progress, calycosin-7-glucoside had never been produced in yeast before. Because its biosynthetic pathway from daidzein is well understood, the compound presents an ideal test case for developing a microbial alternative to plant extraction. Due to these challenges, there is a clear need to develop a robust microbial production route for calycosin-7-glucoside.
A study (DOI: 10.1016/j.bidere.2025.100058) published in BioDesign Research on 24 September 2025 by Jiazhang Lian’s team, Zhejiang University, establishes a yeast-based platform for the de novo biosynthesis of calycosin-7-glucoside, providing a scalable and efficient alternative to plant-derived production of high-value isoflavonoids.
By reconstructing the complete biosynthetic pathway inside Saccharomyces cerevisiae and systematically removing metabolic bottlenecks, researchers created the first yeast platform capable of producing this compound from simple carbon sources.
Stepwise strategy
Using a stepwise metabolic engineering and analytical strategy, the researchers reconstructed and optimized the de novo biosynthetic pathway of calycosin-7-glucoside in Saccharomyces cerevisiae. A previously engineered daidzein-overproducing yeast strain (GYN3) was selected as the chassis, and pathway genes were sequentially integrated to establish the conversion cascade from daidzein to the target glucoside.
Targeted gene knockouts, precursor-supply engineering, enzyme replacement, and gene copy number optimization were systematically applied to relieve metabolic bottlenecks. In parallel, LC–MS-based metabolomics, including selected ion monitoring, product ion fragmentation, and optimized MRM quantification, was employed to identify pathway intermediates and analyze metabolic flux distribution across engineered strains.
READ MORE: Scientists synthesize precursors of powerful anti-cancer drug in yeast cells
READ MORE: Reconstructing nature’s oxindole factory: yeast-based biosynthesis of medicinal indole alkaloids
This integrated approach revealed that introduction of PlOMT9 enabled formononetin formation, while subsequent expression of AmI3′H rapidly converted intermediates to calycosin, indicating high hydroxylase activity. However, incorporation of the native calycosin 7′-O-glucosyltransferase (AmUCGT) resulted in only trace amounts of calycosin-7-glucoside, identifying glycosylation as the primary bottleneck. Deletion of endogenous glucoside hydrolases, particularly EXG1, and enhancement of UDP-glucose supply modestly increased product titers but did not fully resolve the limitation.
Metabolic flux
Replacement of AmUCGT with the more active AmUGT88E29 dramatically shifted metabolic flux toward glycosylated products, increasing the calycosin-7-glucoside to calycosin ratio by over three orders of magnitude. Metabolite profiling further showed that substitution of the glycosyltransferase altered the abundance of multiple side products, highlighting the importance of upstream control points.
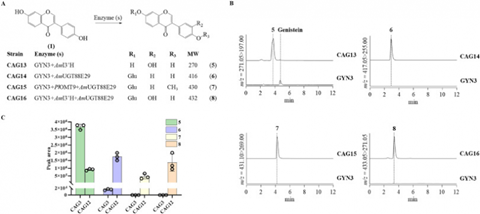
Subsequent gene copy number optimization demonstrated that increasing PlOMT9 dosage most effectively enhanced pathway flux, whereas additional copies of downstream enzymes provided limited benefit. The optimized strain achieved a final calycosin-7-glucoside titer of 0.22 mg/L within 48 hours, establishing PlOMT9 as the pivotal engineering target and underscoring the need for more advanced metabolic control strategies to further improve production.
Proof-of-concept platform
This work establishes a proof-of-concept yeast platform for producing calycosin-7-glucoside and related isoflavonoids. In the long term, such microbial systems could reduce dependence on medicinal plant cultivation, stabilize supply chains, and lower production costs for pharmaceutical and nutraceutical ingredients.
Beyond this single compound, the study provides general design principles—combining metabolomics, enzyme screening, and gene-copy optimization—that can be applied to many other plant-derived natural products.
Topics
- Asia & Oceania
- Astragalus membranaceus
- Bioengineering
- calycosin-7-glucoside
- daidzein
- Disease Treatment & Prevention
- Fungi
- Healthy Land
- Innovation News
- Jiazhang Lian
- Pharmaceutical Microbiology
- Saccharomyces cerevisiae
- Soil & Plant Science
- Strategies for Sustainability
- Sustainable Microbiology
- Synthetic Biology
- Zhejiang University







No comments yet